A metal-free biaryl coupling reaction activated by a sulfonium salt†
Abstract
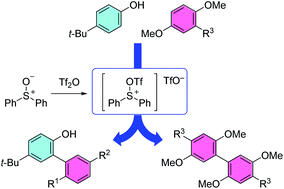
An intermolecular biaryl coupling reaction of phenol derivatives activated by a sulfonium salt formed from diphenyl sulfoxide and trifluoromethanesulfonic anhydride has been developed. The method is a rapid, one-pot reaction leaving no trace of the sulfonium moiety in the coupling products. Tri-substituted phenyl ethers also gave coupling products in moderate yields.


 Please wait while we load your content...
Please wait while we load your content...