Synthesis and anticonvulsant activity of phenoxyacetyl derivatives of amines, including aminoalkanols and amino acids†
Abstract
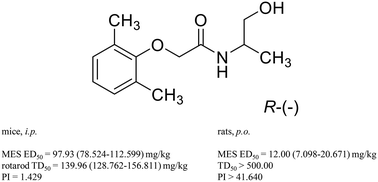
A series of 17 new phenoxyacetamides has been prepared via multistep chemical synthesis as a continuation of the research carried out by our group on di- and tri-substituted phenoxyalkyl and phenoxyacetyl derivatives of amines. The obtained compounds vary in an amide component, for example aminoalkanol or (un)modified amino acid moieties were introduced. The structures of selected products were confirmed by means of crystallographic methods. All 17 compounds were the subject of preliminary screening for potential anticonvulsant activity (MES, 6 Hz and/or scMET tests) and neurotoxicity (rotarod) in mice after intraperitoneal administration, while several active compounds were subsequently examined in additional models (e.g. MES and rotarod – rats, p.o. or i.p., hippocampal kindling – rats, i.p.). Finally, safety studies (cytotoxicity and cell proliferation assays on astrocytes, metabolic stability assessment, mutagenicity evaluation) were performed for several active compounds, including the most promising one (R-(−)-2-(2,6-dimethylphenoxy)-N-(1-hydroxypropan-2-yl)acetamide, MES ED50 = 12.00 mg per kg b.w., rats, p.o.).


 Please wait while we load your content...
Please wait while we load your content...