Synthesis and biological evaluation of 3-(1,3,4-oxadiazol-2-yl)-1,8-naphthyridin-4(1H)-ones as cisplatin sensitizers†
Abstract
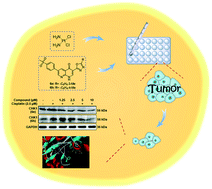
A series of novel 3-(1,3,4-oxadiazol-2-yl)-1,8-naphthyridin-4(1H)-one derivatives were synthesized and their anti-cancer as well as cisplatin sensitization activities were evaluated. Among them, compounds 6e and 6h exhibited significant cisplatin sensitization activity against HCT116. Hoechst staining and annexin V-FITC/PI dual-labeling studies demonstrated that the combination of 6e/6h and cisplatin can induce tumour cell apoptosis. Western blot showed that the expression of ATR downstream protein, CHK1, decreased in 6e + cisplatin and 6h + cisplatin groups compared with that in the test compound and cisplatin group. Furthermore, docking of 6e/6h into the ATR structure active site revealed that the N1 and N8 atoms in the naphthyridine ring and the hybrid atom in the oxadiazole ring are involved in hydrogen bonding with Val170, Glu168 and Tyr155. Additionally, the naphthyridine ring is also involved in π–π stacking with Trp169. Accordingly, compounds 6e and 6h can be expected to be potential cisplatin sensitizers that can participate in HCT116 cancer therapy.


 Please wait while we load your content...
Please wait while we load your content...