Cross-dehydrogenative regioselective Csp3–Csp2 coupling of enamino-ketones followed by rearrangement: an amazing formation route to acridine-1,8-dione derivatives†
Abstract
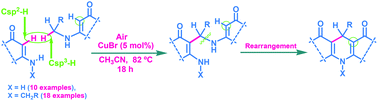
A new general method for the synthesis of acridine-1,8-diones through CDC coupling of enamino-ketones followed by rearrangement has been developed. This is a Cu(I) catalyzed procedure, based on the cross dehydrogenative coupling of the Csp3–H bond with the Csp2–H bond of enamino-ketones followed by rearrangement to acridine-1,8-diones in the presence of PTSA under an aerobic atmosphere. The synthetic route has been broadly applicable to a wide range of enamino-ketone derivatives derived from different benzyl amines as well as primary aliphatic amines having Cα(sp3)–H bonds with various cyclic, acyclic 1,3-diketones and also using DEAD.

 Please wait while we load your content...
Please wait while we load your content...