Fluorescent IGF-II analogues for FRET-based investigations into the binding of IGF-II to the IGF-1R†
Abstract
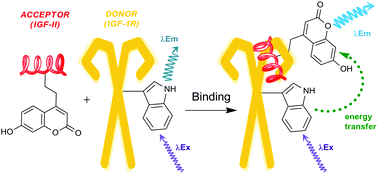
The interaction of IGF-II with the insulin receptor (IR) and type 1 insulin-like growth factor receptor (IGF-1R) has recently been identified as potential therapeutic target for the treatment of cancer. Understanding the interactions of IGF-II with these receptors is required for the development of potential anticancer therapeutics. This work describes an efficient convergent synthesis of native IGF-II and two non-native IGF-II analogues with coumarin fluorescent probes incorporated at residues 19 and 28. These fluorescent analogues bind with nanomolar affinities to the IGF-1R and are suitable for use in fluorescence resonance energy transfer (FRET) studies. From these studies the F19Cou IGF-II and F28Cou IGF-II proteins were identified as good probes for investigating the binding interactions of IGF-II with the IGF-1R and its other high affinity binding partners.

 Please wait while we load your content...
Please wait while we load your content...