A bio-inspired synthetic route to the core ring systems of Spiraea atisine-type diterpenoid alkaloids and related diterpenes†
Abstract
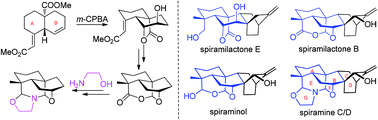
A bio-inspired synthetic strategy for the efficient construction of the structurally complex azapentacyclic ABEFG ring systems of Spiraea atisine-type diterpenoid alkaloids bearing a characteristic internal carbinolamine ether linkage between C(7) and C(20) has been successfully developed. The highly bridged azapentacyclic core structure was constructed rapidly from a readily prepared trans-6,6-bicyclic AB ring precursor through a 14-step sequence. Highlights of the synthesis include a straightforward formal lactone migration from the tricyclic γ-lactone unit of naturally occurring spiramilactone E, and an efficient biomimetic synthesis of the azapentacyclic ABEFG ring systems of spiramines C and D from the corresponding tetracyclic subunits of spiraminol and spiramilactone B.

 Please wait while we load your content...
Please wait while we load your content...