Pd/Al2O3-catalysed redox isomerisation of allyl alcohol: application in aldol condensation and oxidative heterocyclization reactions†
Abstract
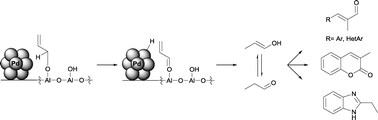
The application of the Pd/Al2O3 catalyst in allyl alcohol isomerization and subsequent aldol condensation and heterocyclization reactions is described. The activity of Pd/Al2O3 in these transformations is suggested to be due to the participation of the Lewis acidic sites of the support in the activation of the alcohol towards oxidative dehydrogenation by the metal and subsequent hydride transfer. The resulting enol(ate)/aldehyde could undergo further reactions promoted by the acid–base properties of the support. In the aldol condensation reactions of the isomerization product, electron poor aromatic aldehydes and heteroaromatic aldehydes showed the highest activity, while aromatic aldehydes bearing electron donating substituents reacted after transformation to the corresponding N-tosyl imines. 1,2-Disubstituted aromatics gave heterocyclic products in one-pot multistep reaction sequences.

 Please wait while we load your content...
Please wait while we load your content...