Pd/C catalysts based on synthetic carbons with bi- and tri-modal pore-size distribution: applications in flow chemistry
Abstract
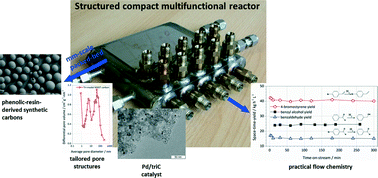
Two new types of phenolic resin-derived synthetic carbons with bi-modal and tri-modal pore-size distributions were used as supports for Pd catalysts. The catalysts were tested in chemoselective hydrogenation and hydrodehalogenation reactions in a compact multichannel flow reactor. Bi-modal and tri-modal micro-mesoporous structures of the synthetic carbons were characterised by N2 adsorption. HR-TEM, PXRD and XPS analyses were performed for characterising the synthesised catalysts. N2 adsorption revealed that tri-modal synthetic carbon possesses a well-developed hierarchical mesoporous structure (with 6.5 nm and 42 nm pores), contributing to a larger mesopore volume than the bi-modal carbon (1.57 cm3 g−1versus 1.23 cm3 g−1). It was found that the tri-modal carbon promotes a better size distribution of Pd nanoparticles than the bi-modal carbon due to presence of hierarchical mesopore limitting the growth of Pd nanoparticles. For all the model reactions investigated, the Pd catalyst based on tri-modal synthetic carbon (Pd/triC) show high activity as well as high stability and reproducibility. The trend in reactivities of different functional groups over the Pd/triC catalyst follows a general order alkyne ≫ nitro > bromo ≫ aldehyde.

 Please wait while we load your content...
Please wait while we load your content...