Investigations on recyclisation and hydrolysis in avibactam mediated serine β-lactamase inhibition†
Abstract
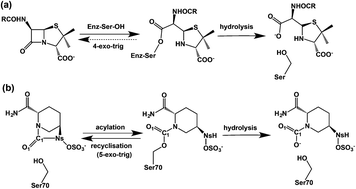
β-Lactams inhibit penicillin-binding proteins (PBPs) and serine β-lactamases by acylation of a nucleophilic active site serine. Avibactam is approved for clinical use in combination with ceftazidime, and is a breakthrough non β-lactam β-lactamase inhibitor also reacting via serine acylation. Molecular dynamics (MD) and quantum chemical calculations on avibactam-mediated inhibition of a clinically relevant cephalosporinase reveal that recyclisation of the avibactam derived carbamoyl complex is favoured over hydrolysis. In contrast, we show that analogous recyclisation in β-lactam mediated inhibition is disfavoured. Avibactam recyclisation is promoted by a proton shuttle, a ‘structural’ water protonating the nucleophilic serine, and stabilization of the negative charge developed on aminocarbonyl oxygen. The results imply the potential of calculations for distinguishing between bifurcating pathways during inhibition and in generating hypotheses for predicting resistance. The inability of β-lactams to undergo recyclisation may be an Achilles heel, but one that can be addressed by suitably functionalized reversibly binding inhibitors.

 Please wait while we load your content...
Please wait while we load your content...