Blocking cyclobutane pyrimidine dimer formation by steric hindrance†
Abstract
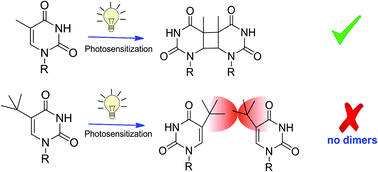
The efficiency of thymine (Thy) and uracil (Ura) to form cyclobutane pyrimidine dimers (CPDs) in solution, upon UV irradiation differs by one order of magnitude. This could to be partially related to the steric hindrance induced by the methyl at C5 in thymine. The aim of the present work is to establish the influence of a bulky moiety at this position on the photoreactivity of pyrimidines. With this purpose, photosensitization with benzophenone and acetone of a 5-tert-butyl uracil derivative (1) and the equivalent Thy (2) has been compared. Introduction of the tert-butyl group completely blocks CPD formation. Moreover, the mechanistic insight obtained by laser flash photolysis is in accordance with the observed photoreactivity.

 Please wait while we load your content...
Please wait while we load your content...