Influence of alkyl chain length on sulfated zirconia catalysed batch and continuous esterification of carboxylic acids by light alcohols†‡§
Abstract
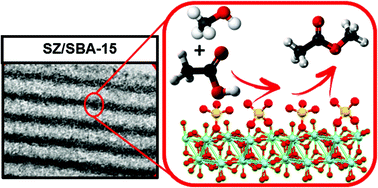
The impact of alkyl chain length on the esterification of C2–C16 organic acids with C1–C4 alcohols has been systematically investigated over bulk and SBA-15 supported sulfated zirconias (SZs). Rates of catalytic esterification for methanol with acetic acid are directly proportional to the sulfur content for both SZ and SZ/SBA-15, with the high dispersion of SZ achievable in conformal coatings over mesoporous SBA-15 confering significant rate-enhancements. Esterification over the most active 0.24 mmol gcat−1 bulk SZ and 0.29 mmol gcat−1 SZ/SBA-15 materials was inversely proportional to the alkyl chain length of alcohol and acid reactants; being most sensitive to changes from methanol to ethanol and acetic to hexanoic acids respectively. Kinetic analyses reveal that these alkyl chain dependencies are in excellent accord with the Taft relationship for polar and steric effects in aliphatic systems and the enthalpy of alcohol adsorption, implicating a Langmuir–Hinshelwood mechanism. The first continuous production of methyl propionate over a SZ fixed-bed is also demonstrated.


 Please wait while we load your content...
Please wait while we load your content...