A pyridinium modified β-cyclodextrin: an ionic supramolecular ligand for palladium acetate in C–C coupling reactions in water†
Abstract
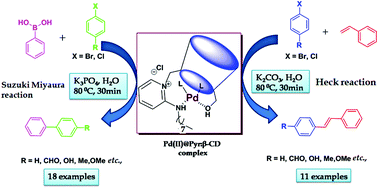
An ionic Pd(II) complex stabilized by a water soluble pyridinium modified β-cyclodextrin was prepared and characterized by NMR, mass spectrometry, FT-IR spectroscopy, UV-visible spectroscopy and DLS (dynamic light scattering). The resulting Pd(II)@Pyr:β-CD complex showed very good catalytic activity in Suzuki–Miyaura and Heck C–C coupling reactions in an environmentally benign water medium. Good to excellent yields were obtained for the coupling of various aryl halides including chlorides with phenylboronic acid/styrene using a catalytic amount of Pd(II)@Pyr:β-CD. This homogeneous catalyst can be reused and recycled more than six times with only marginal loss of its catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...