Gas phase chemical vapor deposition chemistry of triethylboron probed by boron–carbon thin film deposition and quantum chemical calculations†
Abstract
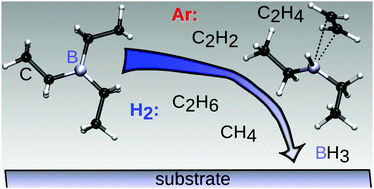
We present triethylboron (TEB) as a single-source precursor for chemical vapor deposition (CVD) of BxC thin films and study its gas phase chemistry under CVD conditions by quantum chemical calculations. A comprehensive thermochemical catalogue for the species of the gas phase chemistry of TEB is examined and found to be dominated by β-hydride eliminations of C2H4 to yield BH3. A complementary bimolecular reaction path based on H2 assisted C2H6 elimination to BH3 is also significant at lower temperatures in the presence of hydrogen. Furthermore, we find a temperature window of 600–1000 °C for the deposition of X-ray amorphous BxC films with 2.5 ≤ x ≤ 4.5 from TEB. Films grown at temperatures below 600 °C contain high amounts of H, while temperatures above 1000 °C result in C-rich films. The film density and hardness are determined to be in the range of 2.40–2.65 g cm−3 and 29–39 GPa, respectively, within the determined temperature window.


 Please wait while we load your content...
Please wait while we load your content...