Mixed systems to assist enzymatic ring opening polymerization of lactide stereoisomers†
Abstract
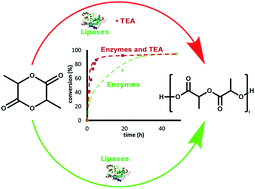
Both D- and L-lactide enantiomers have been selectively enzymatically polymerized by Novozyme 435 and lipase from Burkholderia cepacia, respectively. An optimal temperature was determined for each enzyme (90 and 70 °C, for lipase from Burkholderia cepacia and Novozyme 435, respectively). Various polymerization conditions were tested to improve reaction kinetics and modify the macromolecular architecture. The main results show that enzyme activation by triethylamine, an aprotic amino base, leads to a great improvement in the kinetics (five to six times faster reaction) but also induces slight molar mass variation. Variation of the molar mass is observed for longer reaction times. In a first step, there is an increase possibly due to the activation of a coupling chain reaction, then in a second step a decrease is shown likely due to chain transfer reactions.

 Please wait while we load your content...
Please wait while we load your content...