Computational insight into the mechanism of the Pd(0)–Brønsted acid cooperatively catalysed head-to-tail dimerization of terminal alkynes†
Abstract
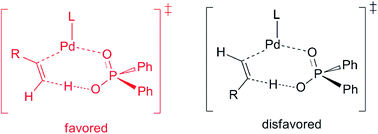
The Pd(0)–Brønsted acid cooperatively catalysed head-to-tail dimerization of terminal alkynes was computationally studied to gain a mechanistic insight. In the presence of the Brønsted acid (Ph2P(O)OH), the formation of a hydridopalladium intermediate via the oxidative addition (OA) of the terminal alkyne Csp–H bond into the Pd(0) centre might not be a favourable reaction pathway. Instead, after the coordination of the Brønsted acid with the Pd(0)–alkyne complex, a proton transfer reaction from the acid to the terminal carbon of the alkyne in a concerted manner is found to be a favourable pathway, leading to the alkenyl(Ph2P(O)O)Pd(II) intermediate. The alternative route that the proton transfer to the internal carbon of alkyne, however, is less competitive. Computational findings suggest that the terminal carbon of the alkyne has more negative charge, and therefore, is more ready to be protonated than the internal carbon in the presence of the Brønsted acid. The regio-selectivity for the proton transfer step is also responsible for the exclusive formation of the head-to-tail dimerization product.

 Please wait while we load your content...
Please wait while we load your content...