Single and double intramolecular proton transfers in the electronically excited state of flavone derivatives†
Abstract
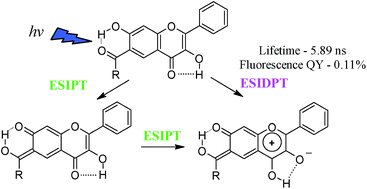
In an attempt to create a flavone derivative able to take part in Excited State Intramolecular Double Proton Transfer (ESIDPT), we synthesized two carbonyl derivatives of 3,7-dihydroxyflavone, both containing two different proton-transfer sites as well as related carbonyl derivatives of 3-hydroxyflavone and 7-hydroxyflavone. All the examined hydroxyflavones were found to participate in the Excited State Intramolecular Proton Transfer (ESIPT). ESIPT which involves 3-hydroxyl and 4-carbonyl groups was found to have a higher barrier compared to ESIPT involving 7-hydroxyl and 6/8-carbonyl fragments. According to the data presented, 3,7-dihydroxy-2-phenyl-6-(3-phenylpropanoyl)-4H-chromen-4-one undergoes a two-stage ESIDPT with formation of an intermediate tautomer. This kind of ESIDPT leads to a tautomeric form with an abnormally low rate of radiative deactivation of the excited state, which conditions low fluorescence quantum yield. The behavior of 3,7-dihydroxy-4-oxo-2-phenyl-4H-chromene-8-carbaldehyde in the electronically excited state is similar to 3-hydroxyflavone derivatives, thus we conclude the occurrence of a single ESIPT in this compound.

 Please wait while we load your content...
Please wait while we load your content...