Self-assembly of dipeptide sodium salts derived from alanine: a molecular dynamics study†
Abstract
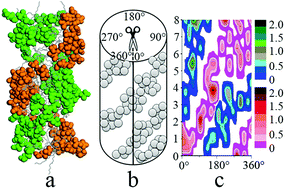
Dipeptide sodium salts derived from alanine can readily self-assemble into helical nanostructures as observed in experiments. But what are the primary driving forces of the organizing process at the molecular level is still worthwhile discussing. In this study, molecular dynamics simulation was employed to investigate the nanostructure at the molecular level, and different driving forces were deduced for the self-assembled process of dipeptide sodium salts. The simulated results showed that van der Waals forces are in favour of the aggregation of alkyl chains in the solution, resulting in one hydrophobic core of condensed fibril. Hydrogen bonds and electrostatic interactions represented by the water-bridge and salt-bridge structure between dipeptide molecules are major driving forces for the hydrophilic amide groups to form the nanostructure shell. In the self-assembly, the bilayer structure of the dipeptide was the basic unit of helical fibril. The structures of the salt bridge and water bridge are distributed over the surface of the fibril, weakening the electrostatic interaction between the dipeptide molecules. The results show that water molecules penetrating into the self-assembled structure should be considered as one part of the peptide self-assembly. Analysis of the self-assembled conformation showed that the hydrophilic amide groups aggregated as small clusters in the hydrophilic shell. Terminal amide groups, forming hydrogen bonds with water molecules around the chiral carbon atom, decide the handedness of the self-assembly.

 Please wait while we load your content...
Please wait while we load your content...