Vinylogous Nicholas reactions in the synthesis of bi- and tricyclic cycloheptynedicobalt complexes†
Abstract
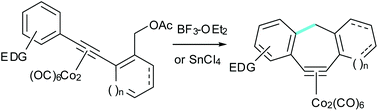
The Lewis acid mediated intramolecular Nicholas reactions of allylic acetate enyne-Co2(CO)6 complexes afford cycloheptenyne-Co2(CO)6 complexes in three manifestations. Electron rich aryl substituted alkyne complexes give tricyclic 6,7,x-benzocycloheptenyne complexes, with x = 5, 6, or 7. Allylsilane substituted complexes afford exo methylene bicyclic x,7-cycloheptenyne complexes (x = 6,7). The allyl acetate function may also be replaced by a benzylic acetate, to afford dibenzocycloheptyne-Co2(CO)6 complexes. Following reductive complexation, the methodology may be applied to the synthesis of the icetexane diterpene carbon framework.

 Please wait while we load your content...
Please wait while we load your content...