Zn(OTf)2 promoted rearrangement of 1,2-cyclopropanated sugars with amines: a convenient method for the synthesis of 3-polyhydroxyalkyl-substituted pyrrole derivatives†
Abstract
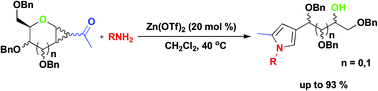
A rearrangement reaction of 1,2-cyclopropanated sugars with alkylamines or arylamines promoted by Zn(OTf)2 is described. The method offers a series of 3-polyhydroxyalkyl-substituted pyrrole derivatives with multiple chiral centers in moderate to excellent yields. The epimerization is achieved by inverting the stereochemistry at the free hydroxyl group of the resulting pyrrole, which would give access to many more possible stereoisomers.

 Please wait while we load your content...
Please wait while we load your content...