A short synthesis of (±)-antroquinonol in an unusual scaffold of 4-hydroxy-2-cyclohexenone†
Abstract
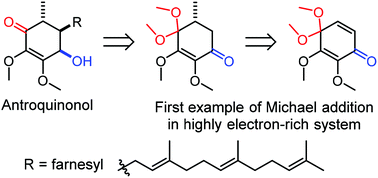
Antroquinonol, which was first isolated from a mushroom, Antrodia cinnamomea, found in Taiwan, is an anticancer compound with a unique core structure of 4-hydroxy-2,3-dimethoxycyclohex-2-enone carrying methyl, farnesyl and hydroxyl substituents in the 4,5-cis-5,6-trans configuration. A short synthesis of (±)-antroquinonol is accomplished in seven steps from 2,3,4-trimethoxyphenol, which is oxidized in methanol to a highly electron-rich substrate of 2,3,4,4-tetramethoxycyclohexadienone and then a Michael reaction with dimethylcuprate is performed as the key step, followed by alkylation, reduction and epimerization to incorporate the required substituents at three contiguous stereocenters.

 Please wait while we load your content...
Please wait while we load your content...