Dendritic polyglycerol sulfate as a novel platform for paclitaxel delivery: pitfalls of ester linkage†
Abstract
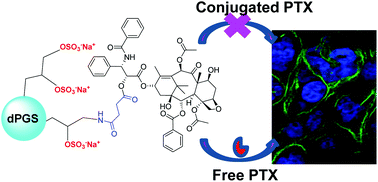
In this study, dendritic polyglycerol sulfate (dPGS) is evaluated as a delivery platform for the anticancer, tubulin-binding drug paclitaxel (PTX). The conjugation of PTX to dPGS is conducted via a labile ester linkage. A non-sulfated dendritic polyglycerol (dPG) is used as a control, and the labeling with an indocarbocyanine dye (ICC) renders multifunctional conjugates that can be monitored by fluorescence microscopy. The conjugates are characterized by 1H NMR, UV-vis measurements, and RP-HPLC. In vitro cytotoxicity of PTX and dendritic conjugates is evaluated using A549 and A431 cell lines, showing a reduced cytotoxic efficacy of the conjugates compared to PTX. The study of uptake kinetics reveals a linear, non saturable uptake in tumor cells for dPGS-PTX-ICC, while dPG-PTX-ICC is hardly taken up. Despite the marginal uptake of dPG-PTX-ICC, it prompts tubulin polymerization to a comparable extent as PTX. These observations suggest a fast ester hydrolysis and premature drug release, as confirmed by HPLC measurements in the presence of plasma enzymes.
- This article is part of the themed collection: Dendritic Polymers for Smart Drug Delivery Applications

 Please wait while we load your content...
Please wait while we load your content...