Syntheses and biological studies of marine terpenoids derived from inorganic cyanide
Abstract
Covering up to 2014
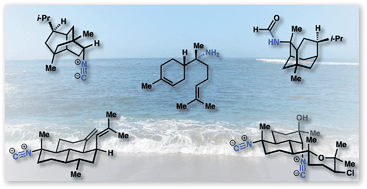
Isocyanoterpenes (ICTs) are marine natural products biosynthesized through an unusual pathway that adorns terpene scaffolds with nitrogenous functionality derived from cyanide. The appendage of nitrogen functional groups – isonitriles in particular – onto stereochemically-rich carbocyclic ring systems provides enigmatic, bioactive molecules that have required innovative chemical syntheses. This review discusses the challenges inherent to the synthesis of this diverse family and details the development of the field. We also present recent progress in isolation and discuss key aspects of the remarkable biological activity of these compounds.

 Please wait while we load your content...
Please wait while we load your content...