Diverse coordination modes in tin analogues of a cyclopentadienyl anion depending on the substituents on the tin atom†‡
Abstract
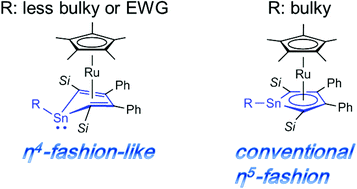
Reactions of an anionic heavy ruthenocene with CCl4, MeI, EtBr and Me3SiCl afforded the first stannole monoanion complexes. Surprisingly, coordination modes of the stannole rings are highly dependent on the substituents on the tin atom. The chloro derivative exhibits a η4-fashion-like coordination mode with a bent stannole ring, whereas the trimethylsilyl derivative adopts the conventional η5-coordination mode. Coordination modes of the alkyl derivatives are in between the two types. Cyclic voltammograms for these complexes reveal that the electron-donating character of the stannole ligand becomes stronger as the stannole ring becomes planar. Theoretical calculations elucidate that the different coordination modes originate from both electronegativity of an adjacent atom to the tin atom and bulkiness of a substituent on the tin atom.

 Please wait while we load your content...
Please wait while we load your content...