Converting between the oxides of nitrogen using metal–ligand coordination complexes
Abstract
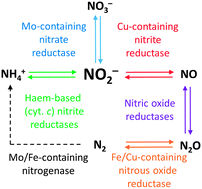
The oxides of nitrogen (chiefly NO, NO3−, NO2− and N2O) are key components of the natural nitrogen cycle and are intermediates in a range of processes of enormous biological, environmental and industrial importance. Nature has evolved numerous enzymes which handle the conversion of these oxides to/from other small nitrogen-containing species and there also exist a number of heterogeneous catalysts that can mediate similar reactions. In the chemical space between these two extremes exist metal–ligand coordination complexes that are easier to interrogate than heterogeneous systems and simpler in structure than enzymes. In this Tutorial Review, we will examine catalysts for the inter-conversions of the various nitrogen oxides that are based on such complexes, looking in particular at more recent examples that take inspiration from the natural systems.

 Please wait while we load your content...
Please wait while we load your content...