Construction of 3,5-dinitrated 1,4-dihydropyridines modifiable at 1,4-positions by a reaction of β-formyl-β-nitroenamines with aldehydes†
Abstract
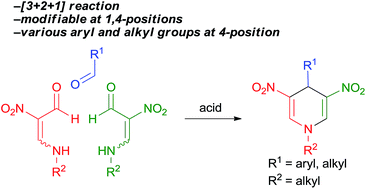
A novel and efficient method for the synthesis of 4-substituted 3,5-dinitro-1,4-dihydropyridines by a reaction of β-formyl-β-nitroenamines with aldehydes was developed. The reaction of nitroenamines with aldehydes leading to 1,4-dihydropyridines and the self-condensation of nitroenamines leading to pyridinium salt intermediate proceed competitively. The obtained 3,4,5-trisubstituted-1,4-dihydropyridines readily transformed into the corresponding pyridines in high yields.

 Please wait while we load your content...
Please wait while we load your content...