A new luminescent Cd(ii)-MOF as a highly selective chemical probe for Fe3+ in aqueous solution with mixed metal ions†
Abstract
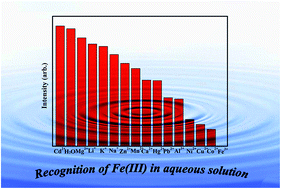
A new metal–organic framework (MOF) with the formula [Cd(H2La)0.5(H2Lb)0.5(H2O)] (1), where H2La2− and H2Lb2− represent two different coordination modes of H2L2− ligands (H4L = [1,1′:4′,1′′-terphenyl]-2′,4,4′′,5′-tetracarboxylic acid), has been synthesized successfully by solvothermal reaction, and characterized by elemental analysis, FT-IR spectroscopy, powder X-ray diffraction (PXRD), and thermogravimetric analysis (TGA). Interestingly, H2L2− adopted two coordination fashions during the self-assembly process of 1 because of the effect of partially deprotonated H4L ligands, which resulted in the 3D framework of 1 showing a trinodal (4,4,4)-connected PtS topology with a point symbol of (42·84). More importantly, the product of 1 displays greatly intense luminescence in the solid state and high sensitivity and selectivity for Fe3+ ions in aqueous solution with mixed ions, making it a new potential probe for detecting Fe3+. The quenching mechanisms are also further discussed in detail.

 Please wait while we load your content...
Please wait while we load your content...