Composition-controlled synthesis of LixCo3−xO4 solid solution nanocrystals on carbon and their impact on electrocatalytic activity toward oxygen reduction reaction
Abstract
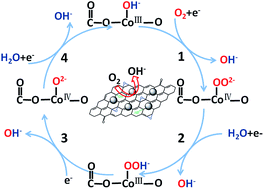
Through incorporation of other metal elements like Li, K and Cu into the Co3O4 lattice, tuning electronic structures of carbon-supported Co3O4 may be an effective way to improve electrochemical properties of the oxide as an efficient catalyst for the oxygen reduction reaction (ORR). In this paper, we have synthesized Li-doped Co3O4 (Li/Co = 0, 2.5%, 5%, 7.5%) solid solution nanocrystals through direct nucleation and growth of the lithium-cobalt oxide on a acid-treated carbon black. The carbon supports LixCo3−xO4 spinel nanocrystals with average particle size of approximately 4 nm, covered evenly on the surface of carbon in this hybrid. The electrocatalysis experiments in an alkaline solution reveal a close correlation between the ORR electrocatalytic activities and the doped Li contents of these LixCo3−xO4/C catalysts. Interestingly, the half-wave potential and the mass-specific activity of these catalysts show a typical volcano plot as a function of Li contents. Among all the synthesized Co3−xO4/C samples, the sample prepared at the Li/Co atomic ratio of 5% displays the most positive half-wave potential and the largest mass-specific activity respectively, which are 70 mV and 3.3 times higher than the undoped Co3O4/C. Compared with that of the undoped Co3O4/C, the remarkly increased content of covalent O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O–CoIII–O bonds formed at the interfaces between Li-doped Co3O4 and carbon support is responsible for the improved electro-catalytic performances of the doped catalyst. Moreover, the covalent electron transfer from the CoIII species to the electron-withdrawing O
C–O–CoIII–O bonds formed at the interfaces between Li-doped Co3O4 and carbon support is responsible for the improved electro-catalytic performances of the doped catalyst. Moreover, the covalent electron transfer from the CoIII species to the electron-withdrawing O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O species through the O
C–O species through the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O–CoIII–O bonds can not only promote the oxidation of active sites CoIII into CoIV but also facilitate the surface hydroxide displacement, which can significantly contribute to the ORR kinetics. Therefore, the exact understanding of the unique interfacial electronic structures of the LixCo3−xO4/C hybrids is very important to develop the lithium-cobalt oxides on carbon as next-generation catalysts for ORR.
C–O–CoIII–O bonds can not only promote the oxidation of active sites CoIII into CoIV but also facilitate the surface hydroxide displacement, which can significantly contribute to the ORR kinetics. Therefore, the exact understanding of the unique interfacial electronic structures of the LixCo3−xO4/C hybrids is very important to develop the lithium-cobalt oxides on carbon as next-generation catalysts for ORR.

 Please wait while we load your content...
Please wait while we load your content...