One-pot, two-step desymmetrization of symmetrical benzils catalyzed by the methylsulfinyl (dimsyl) anion†
Abstract
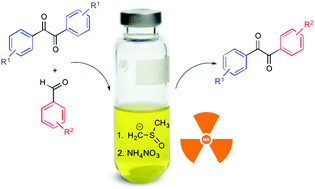
An operationally simple one-pot, two-step procedure for the desymmetrization of benzils is herein described. This consists in the chemoselective cross-benzoin reaction of symmetrical benzils with aromatic aldehydes catalyzed by the methyl sulfinyl (dimsyl) anion, followed by microwave-assisted oxidation of the resulting benzoylated benzoins with nitrate, avoiding the costly isolation procedure. Both electron-withdrawing and electron-donating substituents may be accommodated on the aromatic rings of the final unsymmetrical benzil.

 Please wait while we load your content...
Please wait while we load your content...