Pd(OAc)2/DABCO as an efficient and phosphine-free catalytic system for the synthesis of single and double Weinreb amides by the aminocarbonylation of aryl iodides†
Abstract
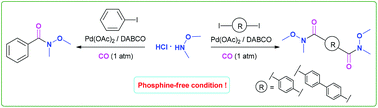
This work reports a mild, stable and efficient Pd(OAc)2/DABCO catalysed protocol for the synthesis of single and double Weinreb amides. Double Weinreb amides, having 1,4-phenylene- and biphenylene-linkers – important backbones for the synthesis of biologically active symmetrical resorcylate oligomer units – were synthesized by the double carbonylation of aryl diiodides. Notably, the reaction does not require any expensive or air/moisture sensitive phosphine ligands. DABCO was found to be an inexpensive and stable ligand for the Pd(OAc)2 catalysed carbonylation of aryl iodides under an atmospheric pressure of carbon monoxide, and offered excellent yields of the single and double Weinreb amides.

 Please wait while we load your content...
Please wait while we load your content...