Mechanistic insight into benzenethiol catalyzed amide bond formations from thioesters and primary amines†
Abstract
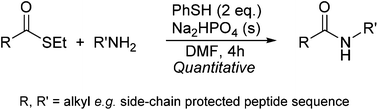
The influence of arylthiols on cysteine-free ligation, i.e. the reaction between an alkyl thioester and a primary amine forming an amide bond, was studied in a polar aprotic solvent. We reacted the ethylthioester of hippuric acid with cyclohexylamine in the absence or presence of various quantities of thiophenol (PhSH) in a slurry of disodium hydrogen phosphate in dry DMF. Quantitative conversions into the resulting amide were observed within a few hours in the presence of equimolar amounts of thiophenol. Ab initio calculations showed that the reaction mechanism in DMF is similar to the well-known aqueous reaction mechanism. The energy barrier of the catalyzed amidation reaction is approximately 40 kJ mol−1 lower than the non-catalyzed amidation reaction. At least partially this can be explained by a hydrogen bond from the amine to the π-electrons of the thiophenol, stabilizing the transition state in the aromatic thioester amidation reaction. Under similar conditions, cysteine-free ligation was achieved by coupling a fully side-chain protected 15 amino acid phosphopeptide thioester to the free N-terminal of a side-chain protected 9 amino acid peptide producing the corresponding 24 amino acid phosphopeptide.

 Please wait while we load your content...
Please wait while we load your content...