Highly efficient oxyfunctionalization of unsaturated fatty acid esters: an attractive route for the synthesis of polyamides from renewable resources†
Abstract
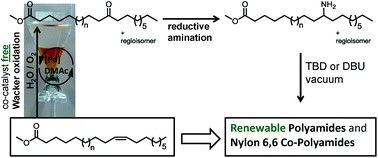
An efficient and environmentally benign strategy for the oxyfunctionalization of fatty acid methyl esters (FAMEs) employing molecular oxygen as an oxidizing agent is described. Keto-fatty acid esters were directly synthesized by co-catalyst-free Wacker oxidations employing oxygen as a sole re-oxidant. Amine functionalization of the thus obtained keto-fatty acid esters was achieved by reductive amination. The prepared renewable AB-type monomers were studied in homopolymerizations as well as in copolymerization reactions with hexamethylendimethylamine and dimethyl adipate to modify the properties of conventional Nylon 6,6. The obtained (co)-polymers were characterized by SEC, NMR and DSC analysis as well as water uptake tests.

 Please wait while we load your content...
Please wait while we load your content...