Carbohydrate-based PBT copolyesters from a cyclic diol derived from naturally occurring tartaric acid: a comparative study regarding melt polycondensation and solid-state modification†
Abstract
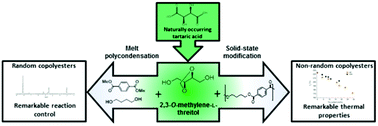
2,3-O-Methylene-L-threitol (Thx) is a cyclic carbohydrate-based diol prepared by acetalization and subsequent reduction of the naturally occurring tartaric acid. The structure of Thx consists of a 1,3-dioxolane ring with two attached primary hydroxyl groups. Two series of partially bio-based poly(butylene terephthalate) (PBT) copolyesters were prepared using Thx as a comonomer by melt polycondensation (MP) and solid-state modification (SSM). Fully random copolyesters were obtained after MP using mixtures of Thx and 1,4-butanediol in combination with dimethyl terephthalate. Copolyesters with a unique block-like chemical microstructure were prepared by the incorporation of Thx into the amorphous phase of PBT by SSM. The partial replacement of the 1,4-butanediol units by Thx resulted in satisfactory thermal stabilities and gave rise to an increase of the Tg values, this effect was comparable for copolyesters prepared by MP and SSM. The partially bio-based materials prepared by SSM displayed higher melting points and easier crystallization from the melt, due to the presence of long PBT sequences in the backbone of the copolyester. The incorporation of Thx in the copolyester backbone enhanced the hydrolytic degradation of the materials with respect to the degradation of pure PBT.

 Please wait while we load your content...
Please wait while we load your content...