Streamlining the conversion of biomass to polyesters: bicyclic monomers with continuous flow†
Abstract
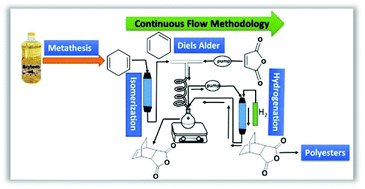
A three-step transformation of 1,4-cyclohexadiene (1,4-CHD) using continuous flow produced an aliphatic bicyclic monomer for polyester synthesis. The monomer synthesis involved catalytic alkene isomerization of 1,4-CHD to 1,3-CHD using a heterogeneous Na2O/Na/Al2O3 catalyst, a Diels Alder reaction with maleic anhydride, and hydrogenation of the bicyclic monomer. A partially continuous strategy was compared with a fully continuous method. The continuous flow process streamlined the transformation of waste by-product biomass by minimizing workup procedures and reducing the synthesis time from ∼1 day for batch processes to ∼2.5 h. The monomer synthesis was easily scalable and allowed recycling of the catalysts for alkene isomerization and hydrogenation. The resulting bicyclic monomers were polymerized with glycerol and 1,4-butanediol (BDO) to obtain renewable polyesters with high thermal stability and tunable glass transition temperatures.

 Please wait while we load your content...
Please wait while we load your content...