Ultrafast cation intercalation in nanoporous nickel hexacyanoferrate†
Abstract
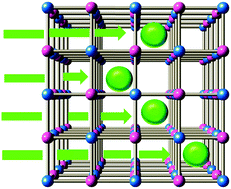
Cation intercalation into nanoporous coordination polymers is utilized in, e.g., Li+/Na+ secondary batteries, the decontamination of radioactive 137Cs+, and so on. Here, we observed an ultrafast intercalation of Na+ and Rb+ within 1500 ms in a thin film of nickel hexacyanoferrate, Na0.68Ni[Fe(CN)6]0.675.0H2O, in aqueous solutions. Quantitative analyses of the intercalation kinetics revealed that the high cation diffusion constant (D ∼ 10−9 cm2 s−1) is responsible for the intercalation.

 Please wait while we load your content...
Please wait while we load your content...