An enantioselective synthesis of the C24–C40 fragment of (−)-pulvomycin†
Abstract
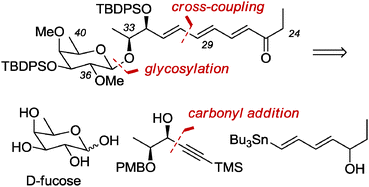
The C24–C40 fragment of (−)-pulvomycin was prepared in enantiomerically pure form using a concise synthesis method (15 linear steps from D-fucose, 6.8% overall yield) featuring a diastereoselective addition to an aldehyde, a β-selective glycosylation and a Stille cross-coupling as the key steps.

 Please wait while we load your content...
Please wait while we load your content...