Low temperature activation of methane over a zinc-exchanged heteropolyacid as an entry to its selective oxidation to methanol and acetic acid†
Abstract
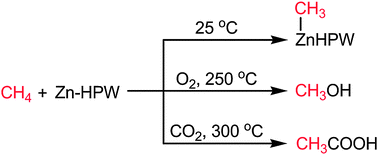
A Zn-exchanged heteropolyacid supported onto silica (Zn-HPW/SiO2) activates methane at 25 °C into Zn–methyl. At higher temperatures and with CH4/O2 or CH4/CO2, it gives methanol and acetic acid respectively.

 Please wait while we load your content...
Please wait while we load your content...