Correlating the hydrogen evolution reaction activity in alkaline electrolytes with the hydrogen binding energy on monometallic surfaces†
Abstract
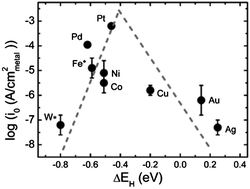
The slow reaction kinetics of the hydrogen evolution and oxidation reactions (HER/HOR) on platinum in alkaline electrolytes hinders the development of alkaline electrolysers, solar hydrogen cells and alkaline fuel cells. A fundamental understanding of the exchange current density of the HER/HOR in alkaline media is critical for the search and design of highly active electrocatalysts. By studying the HER on a series of monometallic surfaces, we demonstrate that the HER exchange current density in alkaline solutions can be correlated with the calculated hydrogen binding energy (HBE) on the metal surfaces via a volcano type of relationship. The HER activity varies by several orders of magnitude from Pt at the peak of the plot to W and Au located on the bottom of each side of the plot, similar to the observation in acids. Such a correlation suggests that the HBE can be used as a descriptor for identifying electrocatalysts for HER/HOR in alkaline media, and that the HER exchange current density can be tuned by modifying the surface chemical properties.

 Please wait while we load your content...
Please wait while we load your content...