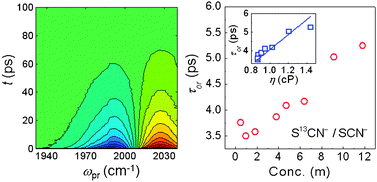
The thiocyanate (SCN−) anion is known as one of the best denaturants, which is also capable of breaking the hydrogen-bond network of water and destabilizing native structures of proteins. Despite prolonged efforts to understand the underlying mechanism of such Hofmeister effects, detailed dynamics of the ions in a highly concentrated solution have not been fully elucidated yet. Here, we used a dispersive IR pump–probe spectroscopic method to study the dependence of vibrational lifetimes and rotational relaxation times of thiocyanate ions on KSCN concentration in D2O. The nitrile stretch mode is used as a vibrational probe for dispersed IR pump–probe and FTIR measurements. To avoid possible self-attenuation of the IR pump–probe signal by highly concentrated SCN− ions, we added a small amount of 13C-isotope-labeled thiocyanate ions (S13CN−) and focused on the excited-state absorption contribution to the IR pump–probe signal of the 13C-isotope-labeled nitrile stretch mode. Quite unexpectedly, the vibrational lifetime of S13CN− ions is independent of the total KSCN concentration in the range from 0.46 m (molality) to 11.8 m while the rotational relaxation time of S13CN− ions is linearly dependent on the total KSCN concentration. By combining the present experimental findings with the fact that the dissolved ions of KSCN salt have a strong tendency to form a large ion cluster in a highly concentrated aqueous solution, we believe that the ion clusters consisting of potassium and thiocyanate ion pairs in D2O behave like ionic liquids and the ions inside ion clusters are weakly bound by electrostatic Coulombic interactions. The ability of SCN− ions to form ion clusters in aqueous protein solutions seems to be a key to understand the Hofmeister ion effect. We anticipate that the present experimental results provide a clue for further elucidating the underlying mechanism of the Hofmeister ion effects on protein stability in the future.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...