Open-shell spherical aromaticity: the 2N2 + 2N + 1 (with S = N + ½) rule†
Abstract
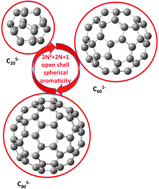
4N Baird's rule represented the extension of Hückel's 4N + 2 rule to triplet state systems. In this work we extend the 2(N + 1)2 Hirsch rule for spherical aromatic species to open-shell spherical compounds and we provide evidence that those spherical species having a same-spin half-filled last energy level with the rest of the levels being fully-filled, i.e., those having 2N2 + 2N + 1 electrons and S = N + ½, are aromatic.

 Please wait while we load your content...
Please wait while we load your content...