Reaction of acetylenic esters and N-functionalized phosphazenes. 1,2- versus 1,4-addition of N-vinylic phosphazenes
Abstract
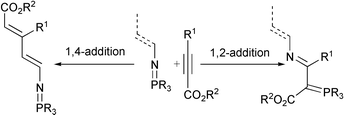
Reaction of phosphazenes derived from aminophosphonates with acetylenic esters leads to conjugated phosphorus ylides. The formation of these stabilized ylides is explained through a [2+2] cycloaddition reaction of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N linkage of the phosphazene (1,2-addition) and the triple bond of the acetylenic ester followed by ring opening of the azaphosphete intermediate. However, in the case of N-vinylic phosphazenes, the phosphazenes derived from triphenyl- and trimethyl-phosphine react as enamines (1,4-addition) with diacetylenic esters, whereas in phosphazenes derived from trimethylphosphine a 1,2-addition of ethyl propiolate to the P
N linkage of the phosphazene (1,2-addition) and the triple bond of the acetylenic ester followed by ring opening of the azaphosphete intermediate. However, in the case of N-vinylic phosphazenes, the phosphazenes derived from triphenyl- and trimethyl-phosphine react as enamines (1,4-addition) with diacetylenic esters, whereas in phosphazenes derived from trimethylphosphine a 1,2-addition of ethyl propiolate to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N linkage of the phosphazene is produced.
N linkage of the phosphazene is produced.

 Please wait while we load your content...
Please wait while we load your content...