Potassium trimethylsilanolate induced cleavage of 1,3-oxazolidin-2- and 5-ones, and application to the synthesis of (R)-salmeterol
Abstract
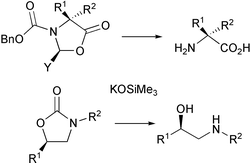
A convenient and efficient method for the cleavage of 1,3-oxazolidin-5-ones and 1,3-oxazolidin-2-ones utilising potassium trimethylsilanolate in tetrahydrofuran is described. The benzyloxycarbonyl-protecting group is readily removed under the reaction conditions, whereas the N-benzoyl group is stable. A synthesis of (R)-salmeterol exploiting the 2-oxazolidinone ring as a protecting group for the ethanolamine moiety is also described.

 Please wait while we load your content...
Please wait while we load your content...