Synthesis of tetrasubstituted pyridines by the acid-catalysed Bohlmann–Rahtz reaction
Abstract
New facile experimental procedures for the preparation of 2,3,4,6-tetrasubstituted pyridines in a single synthetic step have been developed. Thus, an enamino ester and alkynone react by Michael addition–cyclodehydration in a heterocyclisation process that is catalysed by acetic acid, Amberlyst 15 ion exchange resin, zinc(II) bromide or ytterbium(III) triflate. The new one-step Brønsted or Lewis acid-catalysed Bohlmann–Rahtz reaction is a simple, direct and highly expedient method for the synthesis of pyridines that proceeds at a lower reaction temperature and avoids the need to isolate reaction intermediates.
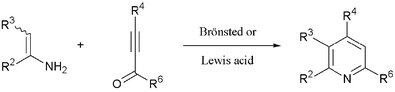

 Please wait while we load your content...
Please wait while we load your content...