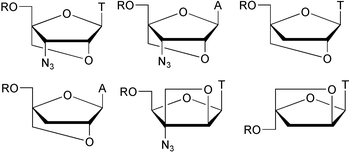
As part of a programme towards evaluating the potential of conformationally locked 3′-deoxy- and 3′-azido-3′-deoxy-nucleoside derivatives as prodrugs of potential 5′-O-triphosphorylated anti-HIV drugs, novel nucleoside derivatives with locked N-type (north-type, C3′-endo) furanose conformation were prepared using convergent synthetic strategies. In addition, masked 5′-monophosphate derivatives of these, and of a conformationally restricted 3′-azido-3′-deoxynucleoside with E-type (eastern-type, O4′-endo) furanose conformation, were prepared in order to potentially circumvent the first phosphorylation step. However, neither the free 5′-hydroxy derivatives nor the masked 5′-monophosphates showed anti-HIV activity in MT-4 cells.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...