N–H Insertion reactions of rhodium carbenoids. Part 3.1 The development of a modified Bischler indole synthesis and a new protecting-group strategy for indoles
Abstract
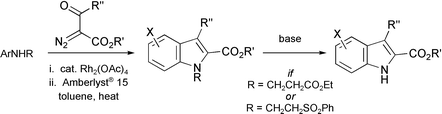
A modified version of the Bischler indole synthesis has been developed in which the key step is the N–H insertion reaction of

 Please wait while we load your content...
Please wait while we load your content...