Nanoporous anodic alumina-based gas diffusion layers for the electroreduction of CO2
María Pilar
Montero-Rama
b,
Domenico
Grammatico
c,
Janine
Lichtenberger
 c,
Virginie
Pellerin
a,
Emilio
Palomares
c,
Virginie
Pellerin
a,
Emilio
Palomares
 de,
Laurent
Billon
de,
Laurent
Billon
 a,
Lluis F.
Marsal
a,
Lluis F.
Marsal
 *be and
Aurelien
Viterisi
*be and
Aurelien
Viterisi
 *a
*a
aThe Institute of Analytical Sciences and Physical Chemistry for Environment and Materials (IPREM), Technopole Hélioparc, 2 Avenue du Président Pierre Angot, 64053, PAU Cedex 09, France. E-mail: Aurelien.viterisi@univ-pau.fr
bDepartament d’Enginyeria Electrònica Elèctrica i Automàtica, Universitat Rovira i Virgili, Avinguda Països Catalans 26, 43007, Tarragona, Spain. E-mail: lluis.marsal@urv.cat
cAIT Austrian Institute of Technology, Center of Energy – Power and Renewable Gas Systems, Giefinggasse 4, 1210, Vienna, Austria
dInstitute of Chemical Research of Catalonia (ICIQ), Avda. Països Catalans 16, 43007, Tarragona, Spain
eICREA, Passeig Lluís Companys 28, E-08010, Barcelona, Spain
First published on 21st November 2025
Abstract
The manufacture of a novel type of gas diffusion electrode (GDE) for the electroreduction of CO2, based on nanoporous anodic alumina gas diffusion layers (GDLs), is described. The GDE consists of an array of aligned pores hydrophobised via silanisation, on top of which a layer of a silver or copper catalyst was deposited. The versatility of the fabrication method allows for controlled pore apertures on both sides of the membrane and controlled thickness, further enabling the tailoring of the GDLs' properties to a given type of catalyst.
Introduction
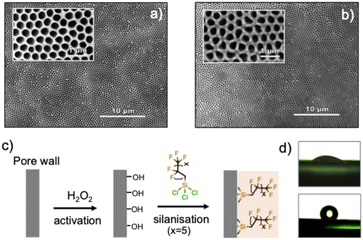
The electrochemical reduction of CO2 into feedstocks, such as permanent gases or alcohols, is a promising alternative for offsetting greenhouse gas emissions.1–34 Recent advances in industry-compatible electrolyser designs5–9 have demonstrated that the reaction can be driven to industry-relevant kinetics. So-called flow cells and membrane electrode assembly (MEA) electrolysers are the most viable systems;7–9 however, their most important component, namely the gas diffusion layer (GDL), is not yet reliable beyond laboratory-scale systems. The porous structure of GDLs addresses the inherent low solubility of CO2 in aqueous media by creating a gas/electrolyte/catalyst interface. This unique configuration allows reaching local CO2 concentrations far beyond its solubility limit, thus enhancing reaction rates.13,14 Early research on gas diffusion electrodes (GDEs), i.e. the combination of a GDL and a catalyst, for the CO2 reduction reaction (CO2RR) focused primarily on carbon-based GDLs.15–19 These types of GDLs provide mechanical stability and electrical conductivity and distribute CO2 gas through their macroscale pores. The conductive carbon fibres are rendered hydrophobic to prevent liquid electrolytes from flooding the gas compartment by incorporating hydrophobising agents into the carbon fibres during the manufacture of the GDL. Despite having some merit,20,21 Sargent and co-workers20 showed that GDLs made from porous PTFE fibres with pore sizes in the 200 to 300 nm range had superior lifetimes than carbon-based GDLs under high electrolysis currents due to the electrically insulating nature of the support. PTFE-based GDLs were shown to perform for 150 hours in either a flow cell or MAE configuration with minimal loss of selectivity at high current densities.22 However, similarly to carbon-based GDLs, PTFE GDLs are challenging to handle. Results strongly suggest that the porosity (pore aperture) dramatically impacts the selectivity, requiring GDLs to be tailored to each catalyst. Indeed, in a recent study, Senocrate and co-workers demonstrated that both intrinsic hydrophobicity and pore size have a capital impact on catalyst selectivity.23 This constitutes a severe limitation of PTFE-based GDLs since neither the arrangement of their pores nor their size can be readily tuned to fit a given catalyst.Therefore, with the aim of tackling the above limitations, herein we report a novel type of GDL manufactured from nanoporous anodic alumina (NAA). The latter material combines the insulating attributes of state-of-the-art Teflon-based GDLs, however, with the added advantages of tuneable porosity and hydrophobicity. NAA, as described, consists of aluminium oxide laminas of variable thickness (from 50 to 300 microns) with a network of a nearly perfect arrangement of nanoscale pores opened at both ends of the cross-section. The membrane was fabricated using a well-established two-step anodisation procedure from aluminium sheets, yielding an aluminium oxide layer with parallel tubular pores that are subsequently freed from the aluminium substrate and opened at the bottom via the selective dissolution of the bottom alumina layer. We produced mechanically robust NAA membranes with an average pore diameter of 350 nm that could readily be hydrophobised via silanisation using perfluorinated agents. Upon the deposition of a catalyst metal such as silver or copper, GDEs with well-defined pore apertures were obtained with demonstrated catalytic activity towards the electroreduction of CO2 in a flow-cell electrolyser. A general scheme of the NAA-GDE fabrication is depicted in Fig. 1.
Results and discussion
The foundation of the work described herein rests on a recent report that demonstrated that NAA could successfully be applied to the diffusion of gases in electrochemical cells. In particular, hydrogen was shown to diffuse efficiently through a Pt-coated NAA membrane in a functioning micro-fuel cell.24 Additionally, the early work of Nam and co-workers showed that nanoporous anodic alumina (NAA) templates could be employed to deposit copper films with varying pore size and depth to be applied to CO2 electroreduction.25 These seminal results suggest that the versatility of NAA membranes and their electrically insulating nature make for a unique candidate that fits the requisites of advanced GDLs.Therefore, we, combined our expertise in NAA fabrication with the electroreduction of CO2 to manufacture NAA-based GDLs following a procedure pioneered by Masuda and co-workers.26 Considering the above requisites and previous work on NAA, it appeared that to be used as GDLs, the optimum pore diameter should be superior to the Knudsen limited diffusion regime while the overall porosity should be the highest possible.16,23,27 According to recent results, a lamina thickness below 200 microns and a pore size slightly superior to 200 nm were deemed sufficient to allow high reaction rates.20 Therefore, a mild anodisation method in phosphoric acid was utilised to establish a benchmark for NAA-GDLs with a self-ordering regime of 195 V yielding NAA with an interpore distance (Dint) of 450 nm. A final porosity of over 60% was targeted, requiring chemical etching in phosphoric acid (4% wt, 35 °C, 90 min) from the as-fabricated NAA layer.28 A schematic of the whole process is depicted in Fig. 1, showing how a 1.5 cm2 area of the NAA layer's backside is exposed through the selective dissolution of the initial aluminium substrate, leaving the so-called residual barrier layer resulting from the NAA growth process on the bottom side.
The latter barrier layer was selectively dissolved using previously described procedures.29,30 A selective dissolution procedure in phosphoric acid (4%) in a two-compartment cell gave membranes with pore openings with similar dimensions at both ends. Experimental parameters such as temperature, acid concentration and experiment time were crucial for providing homogeneous pore openings across the membrane's surface without damaging the pore walls. The set of optimum parameters is compiled in the SI, showing the effect of suboptimal conditions on the final pore shape and size (Fig. S2). Preliminary results showed that, prior to the removal of the barrier layer, the membrane had high mechanical strength when the thickness of the NAA exceeded 50 µm, withstanding shear and compression stresses to the extent that damage would not occur even when dropped from a considerable height. The strength of the membrane decreased significantly after the barrier layer's removal. Therefore, a final thickness of 120 µm was targeted to compensate for this decrease in strength, providing a suitable compromise between pore length and membrane strength. Noteworthily, the manufacturing process leaves an aluminium rim, which serves as a strengthening frame to the NAA membrane and conveniently provides an electrical contact with the metal catalyst subsequently deposited on top of the GDL (Fig. 1b).
After the barrier layer had been removed, the hydrophobicity of the NAA surface was investigated. As seen in Fig. 2d, the surface of the native NAA membranes is intrinsically hydrophilic and must be modified to function as GDLs. Both wet chemical and gas-phase techniques have been described to alter the surface properties of NAA membranes. However, chemical functionalisation using silanisation is by far the most used technique, as it benefits from the fact that the silane precursors form a strong covalent bond with the native oxide surface of the NAA. Alkyl- or perfluoroalkylsilanes, for example, have been shown to increase the membrane's hydrophobicity to a great extent.31–33
Although the degree of hydrophobicity of the GDL may need to be adjusted to each catalyst and electrolyser configuration, the highest possible degree of hydrophobicity was desirable when applying the NAA-GDLs to a flow cell electrolyser. As such, commercially available trichloro(1H,1H,2H,2H-perfluorooctyl)-chlorosilane was used for the NAA functionalisation. In a standard procedure, the native NAA membranes were first activated in a concentrated solution of hydrogen peroxide at 80 °C for 1 h, after which they were dipped in a solution of silane in dry toluene (0.31 g mL−1) for 1 h. After drying at 120 °C overnight, hydrophobic GDLs were obtained. A measure of the contact angle before and after silanisation demonstrated the extent of the shift in surface characteristics, with an initial contact angle of 35° seen to increase to up to 145° in the functionalised NAA (Fig. 2d).
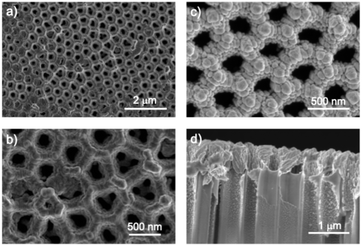
Subsequent to the functionalisation, the formation of the final GDE was achieved by the deposition of the metal catalyst. The latter was deposited as a metallic film of pure silver or copper on the top side of the GDL. Previous results from us and others showed that the deposition of metallic films on NAA surfaces such as gold, platinum, or nickel, using physical vapour deposition methods, leads to highly conductive layers.34–39 Some preliminary trials on the deposition of silver films on top of NAA surfaces were carried out via the latter method; however, DC magnetron sputtering was preferred for consistency with reports on the electrocatalytic reduction of CO2 mediated by metal catalysts. While PVD produced films with a significantly smoother texture (Fig. S3), sputter coating tended to lead to a grainy, penetrating metal coating. In both methods, the metal deposited mainly on the surface of the NAA layer with minimal deposition on the internal pore surface, leaving a sufficiently wide pore aperture.
Silver and copper were investigated for their known selectivity towards CO and mixed adducts. DC magnetron sputtering with an intensity of 80 or 120 W in an argon atmosphere was employed to produce films of Ag and Cu, respectively, with layer thickness varying from 200 to 600 nm. While the static electrical resistance of all the metallic layers was as low as state-of-the-art carbon fibre-based GDLs (below 2 ohm per sq sheet resistance), metallic layers with a thickness of 300 nm had a higher coverage homogeneity across the GDL's surface (Fig. 3).
SEM micrographs of Ag-covered GDLs show a relatively homogeneous metal coverage of the pores' rim. However, pictures of larger areas show sporadic localised inhomogeneities where the pores are overfilled with metal or partially left uncovered (Fig. S4). This effect, presumably due to the hydrophobicity of the NAA, is somewhat limited and was not shown to impact the catalytic properties to a significant extent.
Interestingly, the copper coating was composed of significantly larger metal particles. Overall, the pores' aperture decreases considerably with respect to the pristine GDL, measuring on average 200 nm in both cases, thus remaining large enough for gas to diffuse to the catalyst/electrolyte interface. Both metallic films' surfaces appear grainy, similarly to previous reports of PTFE-coated catalysts, although cross-section micrographs of copper-coated NAA-GDLs show an interesting dendritic-shaped copper layer in the pore's interior. Quite surprisingly, scattered particles of copper are seen to deposit up to 1 µm deep into the pore.
Before submitting the thus-formed GDEs to catalytic conditions, the hydrophobicity of the formed GDE was assessed using contact angle measurements. Quite surprisingly, both metal-covered GDLs were shown to retain most of their original hydrophobicity, experiencing only a slight decrease in contact angle (from 145° to 126° on average, Fig. S8). Thus Ag-GDE were tested as is, while the Cu-based GDEs were further treated with 1-octadecanethiol following a reported procedure,40 which was expected to further maximise the selectivity towards C2 adducts.
The catalytic properties of the GDEs were assessed in a dedicated flow cell system specifically designed to accommodate round-shaped 2 cm diameter NAA-GDE substrates with a 1 cm2 active area. The electrical contact was conveniently provided via the back side of the rim, using spring pins (see the SI for details). Potassium bicarbonate (1 M) was employed as both the catholyte and anolyte, while a standard anion exchange membrane was used to physically separate the respective compartments (Fumasep FAB-PK). A 10 mL min−1 flow rate of CO2 was deemed adequate based on previous results obtained in our group with carbon-based GDEs on a virtually identical flow cell. First trials using partially opened Ag-coated NAA-GDLs confirmed that no catholyte crossing through the GDE to the gas compartment occurred. The selectivity towards CO was somewhat modest, confirming that the high rate of diffusion of CO2 gas through the membrane is critical for keeping the reduction of CO2 kinetically dominant with respect to the hydrogen evolution reaction (HER) (Fig. S5). However, when GDEs with fully opened pores were tested, the silver catalyst faradaic efficiency (FE) towards CO increased significantly. Constant current electrolysis experiments are compiled in Fig. 4a, showing a faradaic efficiency of about 60% up to a 50 mA cm−2 electrolysis current. Higher currents led to the production of substantial amounts of CO, however, with a lower FE, presumably due to the catalyst's passivation. Despite the fact that the current density is lower than state-of-the-art carbon or PTFE-based GDLs, further pore size optimisation will likely match reported FEs.41
The trend in selectivity was somewhat different for Cu-GDEs, showing a substantial amount of hydrogen being produced even at low electrolysis current. A significant amount of formate was also produced at 25 and 50 mA cm−2, and a notable quantity of ethylene and methane was produced at higher current densities (Fig. 4b). Although the FE of carbon-based adducts remained modest, their presence demonstrated that CO2 diffused through the GDL, reaching the catalyst/catholyte interface. The limited selectivity is attributed to the less-than-optimum pore aperture matching with the catalyst, as well as the intrinsic properties of the metal resulting from the sputtering conditions. This is further confirmed by the fact that the FE of H2 remains approximately constant regardless of the current density, indicating that the somewhat modest FE of carbon-based products is not limited by CO2 diffusion but rather by the catalyst's intrinsic properties. However, in both types of GDEs, Ag or Cu, no signs of flooding nor significant delamination were observed after catalysis at 150 mA cm−2. This further indicates that thinner membranes could be used, favouring the kinetics of gas diffusion in and out of the catalytic interface. A long chronopotentiometry experiment was, nonetheless, carried out at 50 mA cm−2 (12 h) to assess the stability of the GDL under continuous electrolysis conditions. The results are depicted in Fig. S7, showing a gradual decrease in selectivity towards CO. Upon disassembly the GDE showed a slight degree of silver delamination, and, while the GDL had not undergone major mechanical alteration, some flooding had occurred. Therefore, the drop in selectivity was partly attributed to catalyst passivation, and gradual flooding.
Conclusion
In the present study, we have demonstrated the applicability of NAA to the manufacture of GDEs for the electrochemical reduction of CO2. This is the first example, to the best of our knowledge, in which an NAA membrane is used as a GDL, allowing CO2 gas to diffuse from one side of the membrane to the other, held in contact with an aqueous electrolyte. Results demonstrated that chemical hydrophobisation was efficient in keeping the catholyte from crossing the membrane during electrolysis and that the deposition of the metal using sputter coating led to catalytically active GDEs with FE towards CO and C2 adducts. A reasonably high selectivity for carbon-based adducts was obtained at low current densities, especially in the case of Ag-GDEs. The modest selectivity at higher current density is presumably due to a less-than-optimum membrane thickness and pore aperture. Quite importantly, no particular care was taken to structure the catalyst to increase its selectivity. As such, these results will serve as the foundation for ongoing studies assessing membrane thickness, pore aperture, and catalyst selectivity. Effort is made to increase the stability of the hydrophobisation layer and to study the diffusion of gas through the porous membrane.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: NAA fabrication, materials characterisation, and electrochemical characterisation. See DOI: https://doi.org/10.1039/d5se01044f.Acknowledgements
The authors thank E2S UPPA, an ANR PIA4 project, for funding the international Chair INTERMAT of E.P. and ENSUITE Hub. This work was also supported by the Spanish Ministeriode Ciencia e Innovación (MICINN/FEDER) PDI2021-128342OB-I00, by the Agency for Management of University and Research Grants (AGAUR) ref. 2021 – SGR-00739, COST Action 20126 – NETPORE and by the Catalan Institution for Research and Advanced Studies (ICREA) under the ICREA Academia Award. Dr Abel Santos is warmly acknowledged for very helpful discussions.Notes and references
- W. A. Smith, T. Burdyny, D. A. Vermaas and H. Geerlings, Joule, 2019, 3, 1822–1834 CrossRef CAS.
- C. Hepburn, E. Adlen, J. Beddington, E. A. Carter, S. Fuss, N. Mac Dowell, J. C. Minx, P. Smith and C. K. Williams, Nature, 2019, 575, 87–97 CrossRef CAS.
- O. S. Bushuyev, P. De Luna, C. T. Dinh, L. Tao, G. Saur, J. van de Lagemaat, S. O. Kelley and E. H. Sargent, Joule, 2018, 2, 825–832 CrossRef CAS.
- F. Franco, C. Rettenmaier, H. S. Jeon and B. Roldan Cuenya, Chem. Soc. Rev., 2020, 49, 6884–6946 RSC.
- E. W. Lees, B. A. W. Mowbray, D. A. Salvatore, G. L. Simpson, D. J. Dvorak, S. Ren, J. Chau, K. L. Milton and C. P. Berlinguette, J. Mater. Chem. A, 2020, 8, 19493–19501 RSC.
- S. Ren, D. Joulié, D. Salvatore, K. Torbensen, M. Wang, M. Robert and C. P. Berlinguette, Science, 2019, 365, 367–369 CrossRef CAS PubMed.
- D. M. Weekes, D. A. Salvatore, A. Reyes, A. Huang and C. P. Berlinguette, Acc. Chem. Res., 2018, 51, 910–918 CrossRef CAS PubMed.
- C.-T. Dinh, T. Burdyny, M. G. Kibria, A. Seifitokaldani, C. M. Gabardo, F. P. García De Arquer, A. Kiani, J. P. Edwards, P. De Luna, O. S. Bushuyev, C. Zou, R. Quintero-Bermudez, Y. Pang, D. Sinton and E. H. Sargent, Science, 2018, 360, 783–787 CrossRef CAS.
- A. Perazio, C. E. Creissen, J. G. Rivera De La Cruz, M. W. Schreiber and M. Fontecave, ACS Energy Lett., 2023, 8, 2979–2985 CrossRef CAS.
- B. Endrődi, G. Bencsik, F. Darvas, R. Jones, K. Rajeshwar and C. Janáky, Prog. Energy Combust. Sci., 2017, 62, 133–154 CrossRef.
- C. Xia, P. Zhu, Q. Jiang, Y. Pan, W. Liang, E. Stavitski, H. N. Alshareef and H. Wang, Nat. Energy, 2019, 4, 776–785 CrossRef CAS.
- D. Gao, P. Wei, H. Li, L. Lin, G. Wang and X. Bao, Acta Phys.-Chim. Sin., 2020, 37, 2009021 Search PubMed.
- P. De Luna, C. Hahn, D. Higgins, S. A. Jaffer, T. F. Jaramillo and E. H. Sargent, Science, 2019, 364, eaav3506 CrossRef CAS.
- T. Burdyny and W. A. Smith, Energy Environ. Sci., 2019, 12, 1442–1453 RSC.
- T. N. Nguyen and C.-T. Dinh, Chem. Soc. Rev., 2020, 49, 7488–7504 RSC.
- S. Hernandez-Aldave and E. Andreoli, Catalysts, 2020, 10, 713 CrossRef CAS.
- K. Liu, W. A. Smith and T. Burdyny, ACS Energy Lett., 2019, 4, 639–643 CrossRef CAS.
- F. Bidault, D. J. L. Brett, P. H. Middleton and N. P. Brandon, J. Power Sources, 2009, 187, 39–48 CrossRef CAS.
- A. Jayakumar, S. P. Sethu, M. Ramos, J. Robertson and A. Al-Jumaily, Ionics, 2015, 21, 1–18 CrossRef CAS.
- C.-T. Dinh, T. Burdyny, M. G. Kibria, A. Seifitokaldani, C. M. Gabardo, F. P. García de Arquer, A. Kiani, J. P. Edwards, P. De Luna, O. S. Bushuyev, C. Zou, R. Quintero-Bermudez, Y. Pang, D. Sinton and E. H. Sargent, Science, 2018, 360, 783–787 CrossRef CAS.
- M. E. Leonard, L. E. Clarke, A. Forner-Cuenca, S. M. Brown and F. R. Brushett, ChemSusChem, 2020, 13, 400–411 CrossRef CAS.
- C. M. Gabardo, C. P. O'Brien, J. P. Edwards, C. McCallum, Y. Xu, C.-T. Dinh, J. Li, E. H. Sargent and D. Sinton, Joule, 2019, 3, 2777–2791 CrossRef CAS.
- A. Senocrate, F. Bernasconi, D. Rentsch, K. Kraft, M. Trottmann, A. Wichser, D. Bleiner and C. Battaglia, ACS Appl. Energy Mater., 2022, 5, 14504–14512 CrossRef CAS.
- W. V. Fernandez, R. T. Tosello and J. L. Fernández, Analyst, 2020, 145, 122–131 RSC.
- K. D. Yang, W. R. Ko, J. H. Lee, S. J. Kim, H. Lee, M. H. Lee and K. T. Nam, Angew. Chem., Int. Ed., 2017, 56, 796–800 CrossRef CAS.
- H. Masuda and K. Fukuda, Science, 1995, 268, 1466–1468 CrossRef CAS PubMed.
- M. Wang, V. Janout and S. L. Regen, Acc. Chem. Res., 2020, 46(12), 2743–2754 CrossRef.
- A. Santos, L. Vojkuvka, M. Alba, V. S. Balderrama, J. Ferré-Borrull, J. Pallarès and L. F. Marsal, Phys. Status Solidi A, 2012, 209, 2045–2048 CrossRef CAS.
- A. Santos, L. Vojkuvka, J. Pallarés, J. Ferré-Borrull and L. F. Marsal, J. Electroanal. Chem., 2009, 632, 139–142 CrossRef CAS.
- M. P. Montero-Rama, A. Viterisi, C. Eckstein, J. Ferré-Borrull and L. F. Marsal, Surf. Coat. Technol., 2019, 380, 125039 CrossRef CAS.
- Z. D. Hendren, J. Brant and M. R. Wiesner, J. Membr. Sci., 2009, 331, 1–10 CrossRef CAS.
- L. Velleman, G. Triani, P. J. Evans, J. G. Shapter and D. Losic, Microporous Mesoporous Mater., 2009, 126, 87–94 CrossRef CAS.
- D. J. Odom, L. A. Baker and C. R. Martin, J. Phys. Chem. B, 2005, 109, 20887–20894 CrossRef CAS PubMed.
- P.-S. Cheow, E. Z. C. Ting, M. Q. Tan and C.-S. Toh, Electrochim. Acta, 2008, 53, 4669–4673 CrossRef CAS.
- S. Y. Lim, C. S. Law, L. Liu, M. Markovic, A. D. Abell and A. Santos, Catal. Sci. Technol., 2019, 9, 3158–3176 RSC.
- G. Jeon, S. Y. Yang, J. Byun and J. K. Kim, Nano Lett., 2011, 5, 1284–1288 CrossRef.
- S. K. Podgolin, D. I. Petukhov, S. G. Dorofeev and A. A. Eliseev, Talanta, 2020, 219, 121248 CrossRef CAS PubMed.
- S.-J. Park, H. Han, H. Rhu, S. Baik and W. Lee, J. Mater. Chem. C, 2013, 1, 5330 RSC.
- B. T. T. Nguyen, E. Z. C. Ting and C.-S. Toh, Bioinspiration Biomimetics, 2008, 3, 035008 CrossRef PubMed.
- D. Wakerley, S. Lamaison, F. Ozanam, N. Menguy, D. Mercier, P. Marcus, M. Fontecave and V. Mougel, Nat. Mater., 2019, 18, 1222–1227 CrossRef CAS PubMed.
- Y. Kim, E. W. Lees and C. P. Berlinguette, ACS Energy Lett., 2022, 7, 2382–2387 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |