Open Access Article
Open Access Article1,8-Naphthalimide-derived reactivity-based fluorescent probes for detection and imaging of H2S
Mannanthara Kunhumon
Noushija
 ,
Alenthwar Vamshi
Krishna
,
Alenthwar Vamshi
Krishna
 and
Sankarasekaran
Shanmugaraju
and
Sankarasekaran
Shanmugaraju
 *
*
Department of Chemistry, Indian Institute of Technology Palakkad, Palakkad-678623, Kerala, India. E-mail: shanmugam@iitpkd.ac.in
First published on 16th January 2026
Abstract
Hydrogen sulfide (H2S) is both an important biological signaling molecule and a toxic environmental pollutant, making its precise detection essential for biomedical research and environmental monitoring. Among the various sensing platforms available, amino-1,8-naphthalimide (Nap)-based fluorescent probes have become powerful tools for real-time H2S detection due to their high sensitivity, excellent selectivity, biocompatibility, and quick response. Nap fluorophores offer several inherent advantages—including strong and tunable emission, prominent intramolecular charge-transfer (ICT) characteristics, large Stokes shifts, and easy structural modification—making them especially attractive as scaffolds for developing activity-based probes. This review highlights recent developments in Nap-derived fluorescent sensors for H2S detection, categorizing the probes based on their reactive sites and sensing mechanisms, such as thiolysis, reduction, and nucleophilic substitution. For each category, we explore structure–function relationships, photophysical properties, sensing performance, and practical applications in biological and environmental settings. Finally, we address current challenges and future directions in designing next-generation Nap-based probes with enhanced ratiometric responses, targeted subcellular localization, two-photon excitation capabilities, and improved suitability for in vivo imaging. Overall, this review offers a comprehensive perspective to guide the rational development of innovative fluorescent tools for accurate and efficient H2S detection.
1. Introduction
Gasotransmitters are a distinct class of endogenously produced gaseous molecules that play pivotal roles in diverse physiological functions.1 The classical gasotransmitter family comprises carbon monoxide (CO), nitric oxide (NO), and hydrogen sulfide (H2S).2 Among them, H2S has attracted particular attention and has historically been recognized for its characteristic odour of rotten eggs and cytotoxic effects. H2S is now appreciated as a biologically relevant signalling molecule with implications in both health and disease.3–6 Notably, unlike CO and NO, H2S exhibits high water solubility, which confers unique biological properties.7 In mammalian systems, endogenous H2S is primarily synthesized through the enzymatic activities of cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3MST).8–11 The abundance of H2S in biological systems varies depending on the enzymatic pathway involved, the organism, the tissue type, and the prevailing physiological state.12 Additionally, bacterial metabolism contributes to H2S production inside the body, while in the environment, H2S is generated as a by-product of several industrial activities, particularly within petroleum-related sectors.13Subsequent research has demonstrated that H2S exerts multiple physiological effects, including regulation of vascular tone, anti-inflammatory activity, neuroprotection, antioxidant defense, and modulation of mitochondrial bioenergetics.2–6,14 These physiological functions highlight the critical role of H2S in maintaining homeostasis and protecting against disorders such as hypertension, neurodegeneration, and cardiovascular disease.11,15–18 In contrast, abnormal H2S levels have been associated with central nervous system diseases, including Down's syndrome, Alzheimer's disease, and Parkinson's disease.19–22 Taken together, current evidence positions H2S as a critical endogenous gasotransmitter with broad physiological relevance and potential therapeutic significance. Therefore, accurate and timely monitoring of H2S levels under both physiological and pathological conditions as well as within biological systems is critically important.
The detection of H2S has traditionally relied on a range of analytical platforms, including chromatographic techniques such as high-performance liquid chromatography (HPLC) and gas chromatography (GC) as well as mass spectrometry, electrochemical sensors, and colorimetric assays.23–27 Although these methods are well established and capable of producing reliable quantitative data, their dependence on sophisticated instrumentation, high operational costs, and time-consuming sample preparation has hindered their broad application, particularly in biological studies.28 Fluorescence-based detection has emerged as a powerful alternative, offering rapid, highly sensitive, and minimally invasive analysis of H2S in complex biological environments.29–31 Unlike conventional methods, fluorescent probes enable real-time monitoring without extensive sample preparation, making them particularly suitable for in situ and live-cell applications. The versatility of organic fluorophores further enhances their utility; their chemical structure can be readily tailored to achieve desirable properties such as selectivity, tuneable emission wavelengths, and biocompatibility.32–36 In cellular imaging, these probes demonstrate clear advantages, including high signal-to-noise ratios, excellent spatial resolution, and compatibility with dynamic biological systems. Over the past decade, a diverse library of fluorescent probes has been developed, each incorporating different fluorophore moieties to optimize sensitivity and selectivity toward endogenous H2S.37–42 Among these fluorophores, Nap has been especially prominent, valued for its robust photostability, ease of structural modification, and broad functional versatility in probe design.43,44 Fluorescent sensing of H2S has primarily evolved along two conceptual pathways. The first involves reaction-based probes, which exploit the distinct reductive properties and strong nucleophilicity of H2S.45–48 The second employs metal-coordination or displacement strategies, where H2S selectively interacts with metal centers to generate a measurable signal.49–52
Among these, reaction-based systems have proven especially advantageous owing to their ease of synthesis, adaptability, and high degree of molecular selectivity. These probes typically rely on H2S-triggered chemical transformations such as the reduction of functional groups (e.g., nitro,45 azide,53 or selenoxide54 moieties), thiolysis reactions involving dinitrophenyl ethers,47 and various nucleophilic substitution or addition reactions (see Scheme 1).55,56 By integrating these mechanisms into fluorogenic scaffolds, researchers have developed probes capable of quantifying H2S with remarkable sensitivity. Importantly, these probes demonstrate strong discrimination of H2S over other biologically relevant sulfur-containing compounds and general reductants, underscoring their utility in complex cellular environments.
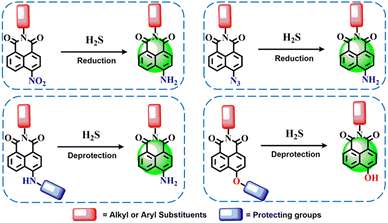 | ||
| Scheme 1 Chemical transformation, such as reduction- and deprotection-based reactivity of Nap fluorescent sensors for H2S detection. | ||
Despite the rapid progress in the development of Nap-derived fluorescent probes, the literature still lacks a dedicated review that exclusively surveys their application in H2S detection and imaging. To address this gap, the present article provides a comprehensive overview of recent advances in Nap-derived small-molecule chemosensors designed for fluorescence monitoring of H2S. Particular emphasis is placed on their structural features, sensing mechanisms, detection strategies, response behaviours, and demonstrated applications. By summarizing the current progress in the field, this review aims to provide researchers with a critical perspective on existing probe designs and to lay the groundwork for developing next-generation fluorescence-based sensing platforms for H2S detection.
2. 1,8-Naphthalimide-based fluorescent probes for H2S
This section discusses the design, synthesis, and reactivity-based fluorescent sensing behaviour of Nap probes toward H2S. For easy understanding and clarity, the Nap probes are categorized into three main classes based on their H2S-triggered chemical transformations: (i) nitro group reduction, (ii) azide group reduction, and (iii) thiolysis-induced deprotection.2.1. H2S sensing by reduction of nitro group
The work by Montoya and Pluth represents a significant early contribution to the field of H2S sensing using the naphthalimide system.45 In this study, a nitro-containing naphthalimide probe Nap-1 was designed to exploit the unique reductive chemistry of H2S, specifically its ability to convert nitro into amine, thereby restoring fluorescence in a non-emissive nitro-naphthalimide scaffold (Fig. 1A). Nap-1 showed significant fluorescence “turn-on” responses at λ = 542 nm after titrating with H2S, exhibiting a 15-fold increase in fluorescence emission within 90 minutes (Fig. 1B). The detection limit was in the range of 5–10 μM. The selectivity of Nap-1 toward H2S was evaluated through titration experiments involving various reactive sulfur, oxygen, and nitrogen species (RSONs), each tested at 100 equivalents (Fig. 1C). For comparison, cysteine and glutathione (GSH) were examined at higher concentrations of 10 mM (2000 equivalents). Notably, Nap-1 displayed a considerable selectivity for H2S compared to other RSONs; however, the addition of 2000 equivalents of cysteine or GSH significantly reduced the selectivity. The biological applicability of Nap-1 was validated using imaging tests in HeLa cells, where the probe effectively revealed the presence of intracellular H2S (Fig. 1D). | ||
| Fig. 1 (A) Chemical structure of Nap-1 and its corresponding amino product formed upon reduction by H2S. (B) Fluorescence “turn-on” response of Nap-1 upon titration with H2S (inset: time-dependent fluorescence enhancement). (C) Relative fluorescence intensity changes of Nap-1 after treatment with various reactive species. (D) Fluorescence imaging of H2S in HeLa cells incubated with 5 μM Nap-1: (a) cells treated with Nap-1 only; (b) cells treated with Nap-1 and H2S. Top: DIC images with nuclear stain overlay. Bottom: fluorescence images. Scale bars = 25 μm. Reproduced with permission from ref. 45. Copyright 2012, Royal Society of Chemistry. | ||
In 2015, Cao and co-workers reported a simple 1,8-naphthalimide fluorescence ‘turn-on’ probe (Nap-2) for the quantitative detection of H2S, through a selective reduction of a nitro group to an amino group, which showed a strong internal charge transfer (ICT) emission at λ = 538 nm (Fig. 2A).57 Upon exposure to H2S in a DMSO/H2O medium, Nap-2 elicited up to 30-fold fluorescence enhancement within a short response time (∼15 minutes). Nap-2 is markedly faster than many previously reported reaction-based H2S sensors (Fig. 2B). The probe displayed a linear fluorescence response for the 0.0 to 100 μM range, making it suitable for quantitative analysis at physiologically relevant H2S concentrations. Selectivity tests confirmed that Nap-2 is highly specific to H2S compared to other common reducing agents and biothiols, with minor interference from cysteine and glutathione (Fig. 2C). The fast kinetics, strong fluorescence enhancement, and high selectivity demonstrated that Nap-2 could serve as a robust and practical probe for biological H2S detection.
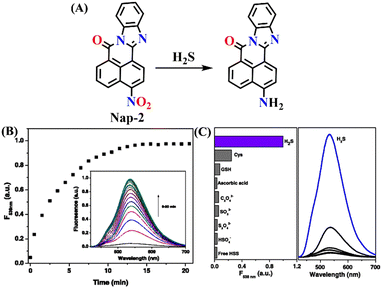 | ||
| Fig. 2 (A) Chemical structure of Nap-2 and its corresponding amino product formed upon reduction by H2S. (B) The fluorescence enhancement observed at λ = 538 nm after incubation with H2S (1.4 × 10−4 M, 10 equiv.) at 25 °C (λex = 409 nm). (C) The selectivity plot demonstrates the high selectivity of Nap-2 for H2S over other analytes. Reproduced with permission from ref. 57. Copyright 2015, Springer. | ||
A study by Velmathi et al. reported an interesting 1,8-naphthalimide-based molecular probe, Nap-3, which showed a rare ability to detect CN−, Fe3+, and particularly H2S with high selectivity (Fig. 3A).58 While CN− and Fe3+ are recognized through a fluorescence relay “on–off” mechanism, H2S was sensed via a chemodosimetric reduction of the nitro group to an amine, producing a distinct red colorimetric response with no fluorescence enhancement (Fig. 3B). This selectivity toward H2S was confirmed by UV-vis absorption, potentiometric titration, and DFT studies, establishing a clear mechanistic basis for the observed color change. The appearance of a new absorption band at λ = 560 nm corroborated the formation of an amine product (Fig. 3C), while potentiometric titrations confirmed the redox interaction. The limit of detection for H2S was determined to be 8.1 μM, a range relevant to biological systems. The probe maintained efficacy across a physiological pH range (7–11), underscoring its potential for biological applications.
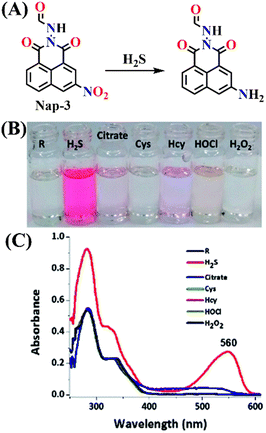 | ||
| Fig. 3 (A) Chemical structure of Nap-3 and its corresponding amino product formed upon reduction by H2S. (B) Naked eye image of H2S selectivity among all RSS and ROS by Nap-3 (labelled as R) via colorimetric output. (C) Electronic absorption response of receptor Nap-3 with all RSS and ROS. Reproduced with permission from ref. 58. Copyright 2020, Royal Society of Chemistry. | ||
In 2025, Sunil and co-workers reported a fluorene–naphthalimide hybrid (Nap-4) as a dual-mode probe that integrates electrochemical and fluorescence-based strategies for highly sensitive detection of H2S.59 The design relied on the incorporation of a nitro-naphthalimide moiety, which acts as both an electron-withdrawing and a reducible recognition unit, conjugated with a fluorene group that enhances photophysical stability. Upon reduction of the nitro group by H2S to an amino group, significant alterations in intramolecular charge transfer occur, leading to marked changes in both the redox profile and the fluorescence response (Fig. 4). Electrochemical studies using square wave voltammetry established a detection limit as low as 12.15 nM at physiological pH, while fluorescence measurements afforded a detection limit of 14 mM. Structural and mechanistic insights were further confirmed through DFT calculations, XPS, and SEM analysis of sulfur deposition. Beyond in vitro sensing, the probe was successfully applied to monitor endogenous H2S in HEK293T cells, demonstrating enhanced fluorescence under nutrient-limited conditions that stimulate autophagy, linking the probe's response to dynamic biological changes in redox homeostasis. Importantly, cytotoxicity assays confirmed good biocompatibility, supporting its applicability in live-cell imaging. This work is significant not only for achieving dual electrochemical and fluorescence detection of H2S with high sensitivity and selectivity but also for demonstrating how molecular probe design can be extended to visualize stress-induced endogenous H2S production, thereby offering a versatile tool for both biomedical and environmental studies.
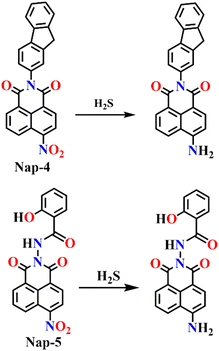 | ||
| Fig. 4 Chemical structure of Nap-4 and Nap-5 and their corresponding amino products formed upon reduction by H2S. | ||
As an extension to this, the same group very recently reported a multifunctional probe, 2-hydroxy-N-(6-nitro-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)benzamide (Nap-5), capable of dual-mode detection of H2S through both optical and electrochemical techniques while also enabling real-time fluorescence imaging of fungal species.60 The probe combines a nitro-naphthalimide fluorophore with a salicyl hydrazide unit to achieve selective H2S sensing via reduction of the nitro group to an amino group, resulting in distinct UV-visible and fluorescence responses (Fig. 4). In UV-visible absorption studies, Nap-5 exhibited a characteristic band at λ = 346 nm that gradually diminished upon addition of Na2S, accompanied by the emergence of a new absorption band at λ = 460 nm with a well-defined isosbestic point at 400 nm, confirming a clean conversion between the two species. A distinct visual colour change accompanied the reduction process, wherein the initially colourless solution gradually developed a brown tint as the concentration of Na2S increased. Fluorescence spectroscopic titration studies further revealed a ratiometric response: the emission peak at λ = 467 nm decreased and a new band at λ = 558 nm emerged with increasing H2S concentration, indicating the formation of the amino derivative. Time-dependent measurements showed the completion of the reaction within 20 hours, yielding a stable fluorescent product. The probe displayed strong selectivity toward H2S over other thiol-containing species and metal ions, along with good photostability and low cytotoxicity. Based on spectroscopic analysis, the detection limit for H2S was estimated to be 13.20 mM. Comprehensive electrochemical, spectroscopic, and theoretical studies established the reduction mechanism and pH-dependent behaviour of Nap-5.
2.2. H2S sensing by reduction of the azide group
While Nap-1 exhibited good selectivity for H2S over reactive sulfur, oxygen, and nitrogen species (RSONs), its performance was compromised in the presence of high concentrations of biologically abundant thiols such as cysteine and glutathione (10 mM, ∼2000 equivalents), which diminished its selectivity. To address this limitation, Pluth designed an azide-based probe, Nap-6, which exhibited a better sensing performance for H2S detection (Fig. 5A).45Nap-6 achieved a rapid and substantial 60-fold fluorescence enhancement within 45 minutes (Fig. 5B) and maintained detection limits in the low micromolar range (1–5 μM). More importantly, Nap-6 displayed markedly higher selectivity for H2S over cysteine and glutathione, even at millimolar concentrations, thereby overcoming a major challenge in the development of biologically relevant H2S probes (Fig. 5C). The applicability of Nap-6 was further demonstrated in live-cell imaging experiments in HeLa cells, where intracellular H2S could be visualized, with Nap-6 producing stronger and more reliable fluorescence responses (Fig. 5D). This pioneering work established the feasibility of using naphthalimide scaffolds for selective, reaction-based H2S sensing. It highlighted how tuning the reducible functionality, from nitro to azide, can significantly enhance selectivity and biological utility. | ||
| Fig. 5 (A) Chemical structure of Nap-6 and its corresponding amino product formed upon reduction by H2S. (B) Fluorescence “turn-on” response of Nap-6 upon titration with H2S (inset: time-dependent fluorescence enhancement). (C) Relative fluorescence intensity changes of Nap-6 after treatment with various reactive species. (D) Fluorescence imaging of H2S in HeLa cells incubated with 5 μM Nap-6: (c) cells treated with Nap-6 only; (d) cells treated with Nap-6 and H2S. Top: DIC images with nuclear stain overlay. Bottom: fluorescence images. Scale bars = 25 μm. Reproduced with permission from ref. 45. Copyright 2012, Royal Society of Chemistry. | ||
Guo et al. reported another interesting example of a naphthalimide-based fluorescent probe (Nap-7) designed for highly selective detection of H2S in aqueous environments, based on an azide-to-amine reduction strategy (Fig. 6A).61 The probe Nap-7 was synthesized via a simple two-step route and functionalized with a hydroxyl diethyl ether substituent to enhance water solubility, making it suitable for environmental applications. In buffered aqueous DMSO medium, Nap-7 exhibited weak fluorescence (Φ = 0.012), but in the presence of Na2S, a robust fluorescence “turn-on” response was observed, with a more than 25-fold intensity increase and good linearity in the range of 0–40 μM (Fig. 6B). The detection limit was found to be as low as 0.37 μM by fluorescence measurements, and 1.4 ppb in water samples after a simple pre-treatment, highlighting its excellent sensitivity. Importantly, Nap-7 displayed remarkable selectivity, showing negligible interference from a wide range of reactive sulfur, oxygen, and nitrogen species (RSONs), anions, cations, and ROS, even at concentrations much higher than that of H2S (Fig. 6C). The response was also rapid, reaching completion within 15 minutes, making it suitable for practical monitoring. Application to real water samples (tap, mineral, and lake) showed excellent recoveries ranging from 94% to 108%, with low relative standard deviations, confirming the method's analytical reliability. This work not only reinforced the utility of azide-to-amine reduction chemistry for H2S recognition but also extended the use of naphthalimide scaffolds beyond biological systems into environmental monitoring, setting a precedent for designing selective, water-compatible probes for gasotransmitter detection.
 | ||
| Fig. 6 (A) Structure of Nap-7 and its amino product formed after reduction by H2S. (B) Fluorescence spectra (λex = 440 nm) of Nap-7 (10 μM) with 0–100 μM Na2S (10 equiv.) in 20 mM pH 7.4 HEPES buffer (containing 50% DMSO). The inset is the corresponding relationship between fluorescence intensity and 0–300 μM Na2S (30 equiv.) (λex = 440 nm, λex = 534 nm). (C) Fluorescence response of Nap-7 towards different analytes ((1) 100 μM Na2S, (2) 100 μM SO32−, (3) 100 μM S2O32−, (4) 100 μM S2O42−, (5) 1 mM SCN−, (6) 1 mM SO42−, (7) 1 mM Cl−, (8) 1 mM Br−, (9) 1 mM I−, (10) 1 mM NO3−, (11) 1 mM NO2−, (12) 1 mM HPO42−, (13) 1 mM HCO3−, (14) 1 mM ClO3−, (15) 1 mM OAc−, (16) 1 mM citrate, (17) 1 mM Co2+, (18) 100 mM HSO3−, (19) 100 mM S2O72−, (20) 1 M ClO3−, (21) 1 M H2O2, and (22) 1 M CrO3). Reproduced with permission from ref. 61. Copyright 2014, Royal Society of Chemistry. | ||
In 2016, Choi and co-workers advanced the concept of azide reduction further by reporting two naphthalimide-based probes (Nap-8 and Nap-9) for H2S detection, designed with structural variations at the N-imide site to tune the solubility and sensing performance (Fig. 7A).62 Both probes responded selectively to H2S by converting the azide group into an amine, restoring ICT and yielding strong fluorescence “turn-on” signals. Among the two probes, Nap-8 exhibited much stronger sensing performance than Nap-9. When treated with H2S, Nap-8 produced nearly a 70-fold fluorescence enhancement at λ = 550 nm (Fig. 7B, inset). It also achieved a high quantum yield of 0.72 and a very low detection limit of less than 0.3 μM in PBS buffer. In addition, Nap-8 reacted with faster kinetics compared to Nap-9, making it the more efficient probe. Importantly, Nap-8 displayed excellent selectivity against other reactive species and biothiols as well as strong water solubility without the need for organic co-solvents (Fig. 7B).
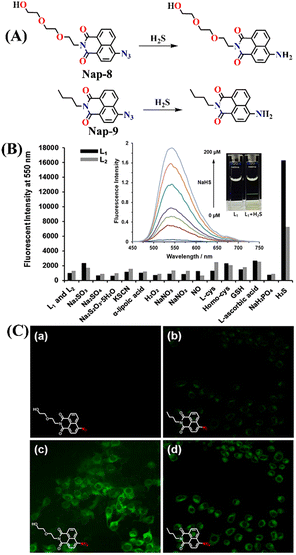 | ||
Fig. 7 (A) Structure of Nap-8 and Nap-9 and their amino product formed after reduction by H2S. (B) Fluorescence responses of Nap-8 and Nap-9 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μM) toward various analytes and NaHS (100 μM) toward various analytes and NaHS (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μM) in PBS buffer (pH 7.4) at 37 μM) in PBS buffer (pH 7.4) at 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 60 °C for 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min. Excitation at 435 min. Excitation at 435![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm (inset: time-dependent fluorescence spectra of Nap-8 (10 μM) with NaHS in PBS (pH 7.4) at 37 °C for 30 min, and visual color changes observed). (C) Fluorescence images of exogenous H2S in living RAW264.7 cells incubated with Nap-8 and Nap-9. Cells were incubated with only 5 μM probes for 30 min (a and b) and with probes for 30 min and then 200 μM NaHS for 5 min (c and d), under λ = 488 nm excitation. Images were acquired with emission channels of λ = 505–605 nm (green). Reproduced with permission from ref. 62. Copyright 2016, Nature Portfolio. nm (inset: time-dependent fluorescence spectra of Nap-8 (10 μM) with NaHS in PBS (pH 7.4) at 37 °C for 30 min, and visual color changes observed). (C) Fluorescence images of exogenous H2S in living RAW264.7 cells incubated with Nap-8 and Nap-9. Cells were incubated with only 5 μM probes for 30 min (a and b) and with probes for 30 min and then 200 μM NaHS for 5 min (c and d), under λ = 488 nm excitation. Images were acquired with emission channels of λ = 505–605 nm (green). Reproduced with permission from ref. 62. Copyright 2016, Nature Portfolio. | ||
The biological applicability of Nap-8 and Nap-9 was validated through confocal fluorescence imaging in RAW264.7 macrophage cells. Both probes were cell-permeable and minimally cytotoxic (>90% cell viability at 20 μM after 24 h). Cells treated with Nap-8 alone showed negligible background fluorescence, but a bright green emission was observed after exposure to NaHS, with Nap-8 showing much stronger intensity due to its enhanced solubility and cellular retention (Fig. 7C). Extending to in vivo models, Nap-8 successfully visualized endogenous H2S in zebrafish embryos, with signals localized in the yolk, brain, and spinal cord, and its specificity confirmed by aminooxyacetic acid (AOAA) inhibition experiments. Overall, this study demonstrates how modifying the structure of naphthalimide scaffolds can significantly enhance solubility and sensitivity, with Nap-8 serving as a powerful probe for both cellular and whole-organism imaging of H2S.
In 2020, Lin et al. reported an ICT-based naphthalimide fluorescent probe (Nap-10) incorporating a bridging Si–O–Si unit for the selective visualization of H2S in lipid droplets of living cells (Fig. 8A).63 The probe design employs an azide-functionalized 1,8-naphthalimide as the H2S-responsive unit, wherein H2S-triggered reduction of the azide to the corresponding amine converts an electron-withdrawing group into a strong electron donor. This transformation effectively activates the ICT process, resulting in a prominent fluorescence turn-on response. The introduction of an organosilicone (Si–O–Si) linker not only improves biocompatibility but also facilitates preferential localization in lipid droplets, thereby enabling subcellularly resolved H2S imaging. Probe Nap-10 exhibits a characteristic ICT-driven spectral response toward H2S. In the absorption spectra, the addition of H2S induces a noticeable red shift accompanied by the appearance of a new absorption band around λ = 440 nm, consistent with enhanced ICT following azide-to-amine reduction (Fig. 8B). Correspondingly, the fluorescence spectra display a strong turn-on emission centered at approximately λ = 550 nm, with the emission intensity increasing linearly over a relevant H2S concentration range and reaching saturation within minutes, indicative of rapid reaction kinetics (Fig. 8C). Notably, the probe shows minimal interference from common biological anions, thiols, and metal ions, underscoring its high selectivity for H2S (Fig. 8D). Beyond solution studies, probe Nap-10 demonstrated favorable biological performance, including low cytotoxicity, good photostability, and efficient cellular uptake. Importantly, the presence of the Si–O–Si linker confers pronounced lipid droplet targeting, as evidenced by strong co-localization with Nile Red in HeLa cells (Fig. 8E).
 | ||
| Fig. 8 (A) Structure of Nap-10 and its amino product formed after reduction by H2S (photographs imaged at different equivalents of H2S under light illumination). (B) The absorption spectra of Nap-10 in the presence of different analytes and H2S (color of the sensor before and after H2S addition). (C) Fluorescence spectra of Nap-10 in aqueous solution upon addition of different concentrations of H2S. (D) Fluorescence response of Nap-10 towards different analytes in aqueous solution. (E) Fluorescence intensity monitored at λ = 550 nm of Nap-10 in the presence of different ions ((1) Nap-10, (2) Cys, (3) D-Cys, (4) EtOONH4, (5) Glu, (6) Gly, (7) KNO3, (8) NaNO2, (9) Na2S2O3, (10) NaBr, (11) NaCl, (12) NaClO, (13) NaCNS, (14) NaF, (15) NaHCO3, (16) NaI, (17) Na2SO3, (18) Na2SO4, (19) Na2CO3, (20) Na2S2O4, and (21) H2S) in aqueous solution, λex = 405 nm. (F) Co-localization of Nap-10 and Nile Red neutral lipid stains in HeLa cells, (a) merged image of (b)–(d); (b) green channel λex = 405 nm, collected 500–550 nm; (c) Nile Red neutral lipid stains, λex = 561 nm, collected 570–620 nm; (d) bright field. Reproduced with permission from ref. 63. Copyright 2020, Royal Society of Chemistry. | ||
Iyer and co-workers reported a novel probe (Nap-11) constructed by linking two azide-functionalized naphthalimide units via an ethylene bridge bearing two cyano substituents (Fig. 9A).64Nap-11 utilizes the reduction of an azide group to an amine for highly selective “turn-on” detection of hydrogen sulfide in both environmental and biological samples. The probe exhibited weak background emission due to PET quenching, but upon exposure to H2S, a strong green fluorescence at λ = 452 nm was rapidly restored with significant emission enhancement as the azide was reduced to an electron-donating amine (Fig. 9B). Nap-11 demonstrated an excellent selectivity over other anions, reactive oxygen/nitrogen species, and biothiols (Fig. 9C), and showed a linear response up to 5 μM H2S with a low detection limit of 1.5 μM. Selective sensing of H2S was also reflected by a marked color change from colourless to bright green emission (Fig. 9D). The response was fast, reaching completion within 2 seconds, and fluorescence intensity remained stable across a broad pH range (4–10), highlighting the probe's suitability for real-time monitoring. Quantum yield analysis further revealed a ∼5.3-fold increase upon reduction, confirming the efficiency of the sensing mechanism. Practical applicability was validated by detecting H2S in environmental matrices (tap water, lake water, well water, beer, and red wine), with recovery rates between 87% and 94% and RSD values below 1.5%, demonstrating analytical reliability. Moreover, Nap-11 successfully penetrated HeLa cells and enabled fluorescence bioimaging of intracellular H2S, showing strong green fluorescence only after sulfide treatment, with negligible background in controls (Fig. 9E). Importantly, compared to earlier multistep and slower systems, Nap-11 offers a simple synthetic route, ultrafast response, and practical dual applicability, reinforcing the value of azide-to-amine chemistry in the ongoing development of naphthalimide-based H2S sensors.
 | ||
| Fig. 9 (A) Structure of Nap-11 and its amino product formed after reduction by H2S. (B) Change in fluorescence intensity of Nap-11 (2 μM) over the incremental addition of H2S (up to 5 equiv.; inset: plot of fluorescence intensity against the concentration of H2S). (C) Fluorescence spectra of probe Nap-11 (labelled as NAM-N3) (2 μM) upon addition of various analytes (10 equiv.). (D) Photographs of Nap-11 in the presence of various anions and H2S under a UV lamp at 365 nm. (E) Bright-field and fluorescence images of HeLa cells treated with Nap-11 and H2S; (a) HeLa cells alone, (b) HeLa cells treated only with Nap-11 (labelled as NAM-N3), (c) HeLa cells treated with Nap-11 (labelled as NAM-N3) and H2S. The scale bar is 50 μm. Reproduced with permission from ref. 64. Copyright 2021, Elsevier. | ||
Later, the same group developed another probe, Nap-12, incorporating a pyridylmethyl substituent at the imide site of the naphthalimide ring based on the same principles, for the selective detection of H2S in aqueous and biological samples (Fig. 10A).65 Spectroscopic analysis of Nap-12 showed a distinct absorption shift from λ = 365 to 416 nm with a well-defined isosbestic point at λ = 391 nm. This spectral change was accompanied by a visible transition in solution color, from colourless to yellow, enabling both straightforward colorimetric readout and fluorescence-based sensing. Upon reduction by H2S, the probe displayed a pronounced emission at λ = 459 nm with an approximately 5.3-fold enhancement in quantum yield (Fig. 10C). It showed a linear response in the 0–10 μM range, a low detection limit of 1.2 μM, and a rapid response time of just 8–10 seconds; outperforming many previously reported naphthalimide-based sensors. The selectivity was excellent, with negligible interference from a wide range of competing anions, ROS/RNS, or biothiols, and the fluorescence signal remained stable under physiological pH conditions (7–12) (Fig. 10B). For practical demonstration, Nap-12 was tested in diverse real-world matrices, including tap water, lake water, well water, beer, and red wine, where it delivered recoveries of 89–97% with relative standard deviations under 1.6%. In addition, simple test strips coated with the probe showed a clear visual change from colorless to green in the presence of H2S, highlighting its potential for portable and on-site applications (Fig. 10D). Biological imaging experiments further confirmed its utility: in E. coli cells, Nap-12 readily penetrated and produced bright fluorescence signals upon H2S exposure, enabling clear visualization of intracellular sulphide (Fig. 10E).
 | ||
| Fig. 10 (A) Structure of Nap-12 and its amino product formed after reduction by H2S. (B) Change in fluorescence intensity of Nap-12 (20 μM) in the presence of various analytes and (C) fluorescence changes on the incremental addition of H2S (0 and 80 μM). (D) Photograph showing fluorescence color change of Nap-12 (2 × 10−5 M) coated test paper strips after treating with various anions and small molecules. (E) (a) Fluorescence bioimages of E. coli bacterial cells alone, (b) E. coli cells treated with Nap-12, (c) E. coli cells treated with Nap-12 and H2S using the blue channel. (d) E. coli cells were treated with Nap-12 in the presence of H2S ion using the green channel. Reproduced with permission from ref. 65. Copyright 2022, Elsevier. | ||
Yan and co-workers developed another simple naphthalimide-based fluorescent probe (Nap-13) for H2S detection (Fig. 11A).66 The probe was synthesized in just two steps from readily available precursors, making it more cost-effective compared to many previously reported systems, and its structure and sensing mechanism were confirmed through NMR, IR, ESI-MS, and theoretical calculations. In its azide form, probe Nap-13 was essentially non-fluorescent; however, upon exposure to H2S, it displayed a pronounced fluorescence enhancement with a strong red emission centered at λ = 510 nm (Fig. 11B) and a distinct color change under UV light in DMSO, enabling both visual and spectroscopic detection (Fig. 11B, inset). Sensitivity studies demonstrated a detection limit as low as 1 × 10−7 M. Selectivity tests revealed negligible interference from common competing species, including cations, anions, reactive sulfur and oxygen species, and biothiols such as cysteine and glutathione, indicating the probe's robustness in complex environments (Fig. 11C). Importantly, the probe was effectively applied in biological imaging. Confocal microscopy in SiHa cells revealed that the probe is cell-permeable and produces strong intracellular fluorescence upon exposure to H2S (Fig. 11D). Moreover, it exhibited low cytotoxicity, maintaining over 80% cell viability even at concentrations up to 60 μM, underscoring its excellent biocompatibility. These results position probe Nap-13 as a versatile and practical addition to the family of azide-functionalized naphthalimide sensors for H2S detection and imaging.
 | ||
| Fig. 11 (A) Structure of Nap-13 and its amino product formed after reduction by H2S. (B) Fluorescence spectral changes of Nap-13 (4 μM) upon addition of Na2S (0.0–0.70 μM) in DMSO at room temperature (inset: the visible fluorescence changes upon UV irradiation). (C) Fluorescence response of Nap-12 upon the addition of several ions in DMSO. (D) Bright-field and fluorescence images of SiHa cells treated with Nap-13 and H2S. Reproduced with permission from ref. 66. Copyright 2022, Springer. | ||
Wang and co-workers systematically advanced naphthalimide-based H2S probes by progressing from a single ICT-regulated design to a synergistic ICT-PET dual-controlled strategy, thereby significantly enhancing sensitivity and biological imaging performance. In their earlier study (reported in 2020),67 an azide-functionalized naphthalimide probe (Nap-14) displayed typical ICT-dominated behavior, wherein H2S-induced azide-to-amine conversion produced a red shift in the absorption spectrum along with a pronounced fluorescence turn-on in the green region, enabling rapid and selective H2S detection under pH-controlled conditions (Fig. 12). Building on this design, a subsequent probe (Nap-15) was developed by introducing a PET-active p-hydroxyphenyl unit, which imposed dual quenching (ICT + PET) in the ground state and resulted in an exceptionally low background fluorescence (Fig. 12).68 Upon exposure to H2S or visible-light irradiation, azide reduction effectively suppressed the PET process while simultaneously activating ICT, leading to a strong fluorescence enhancement of approximately 17-fold and a markedly improved detection limit of 0.085 μM, representing an order-of-magnitude improvement over the single ICT-based probe. Spectral studies revealed that both probes (Nap-14 and Nap-15) exhibit red-shifted absorption bands in the range of ∼430–460 nm and turn-on emission centered at ∼520–540 nm, consistent with a common ICT-driven signaling mechanism. Importantly, the ICT-PET dual-regulated probe Nap-15 enabled spatiotemporal control over fluorescence activation and facilitated high-contrast imaging of exogenous H2S in living HeLa cells, characterized by an excellent signal-to-noise ratio and minimal background interference. Collectively, these results underscore a clear design evolution in which the rational integration of multiple photophysical quenching pathways substantially improves H2S sensing sensitivity and broadens applicability toward precise and photoactivatable cellular imaging.
 | ||
| Fig. 12 Structures of probe Nap-14 and Nap-15 and their amino product formed after reduction by H2S. | ||
Zhang and co-workers reported another simple azide-functionalized fluorescent probe (Nap-16), structurally closely related to probe Nap-15, that operates via the selective H2S-mediated reduction of an aryl azide to the corresponding amine.69 In its native azide form, probe Nap-16 is weakly emissive; however, exposure to H2S induces the emergence of a new absorption band in the visible region along with a pronounced fluorescence turn-on in the green region (Fig. 13A). The fluorescence intensity increases in a concentration-dependent manner, delivering a low detection limit of 17.4 nM and a rapid response time within minutes. The probe demonstrates high selectivity for H2S over common anions, biothiols, and reactive oxygen and sulfur species across a broad pH range. Biological studies revealed low cytotoxicity and efficient cellular uptake, enabling successful fluorescence imaging of exogenous H2S in living cells. Notably, the applicability of probe 4 was further extended to food safety monitoring, where it facilitated the visual detection of H2S released during food spoilage through observable fluorescence changes in real samples, such as red wine. Collectively, these findings illustrate that azide-reduction-based fluorescent probes offer reliable spectroscopic responses while being readily adaptable to both bioimaging and practical food-quality assessment.
 | ||
| Fig. 13 (A) Structure of Nap-16 and its amino product formed after reduction by H2S. (B) The absorption and (C) fluorescence emission spectra of Nap-16 in the presence of increasing concentrations of H2S (inset: color changes observed). (D) Photograph of the luminescent test paper exposed to various anions imaged under a UV lamp. Reproduced with permission from ref. 69. Copyright 2020, Elsevier. | ||
In 2024, Széchenyi et al. reported a new azide-functionalized naphthalimide probe (Nap-17) for the highly sensitive detection of H2S in human serum (Fig. 14).70 The probe combined a 1,8-naphthalimide fluorophore with a p-toluidine substituent at the imide position, and an azide group served as the H2S-responsive recognition site. This modification enhanced the photophysical properties of the scaffold, improving fluorescence output and stability under physiological conditions. The probe itself was weakly emissive, but in the presence of H2S it underwent selective azide-to-amine reduction, leading to a pronounced “turn-on” fluorescence at λ = 520 nm. Spectral studies confirmed an excellent linear correlation between fluorescence intensity and H2S concentration in the low micromolar range, and the detection limit was calculated to be 0.085 μM, which is lower than that of many earlier naphthalimide-based systems. Selectivity tests demonstrated that the probe responded exclusively to H2S even in the presence of other biologically abundant anions and reactive sulfur species, underscoring the specificity of the azide reduction pathway. Practical validation was performed in human serum, where spiking experiments with 5–20 μM H2S yielded recoveries of 95–109% and low relative standard deviations, confirming the probe's analytical reliability in complex biological environments. Importantly, the endogenous H2S concentration in a real serum sample was quantified at 17.2 μM, consistent with physiologically reported levels, highlighting the probe's biomedical relevance. The requirement of two equivalents of H2S for complete reduction of the azide moiety was also experimentally verified, aligning with the known mechanistic pathway.
 | ||
| Fig. 14 Structures of probe Nap-17 and Nap-18 and their amino product formed after reduction by H2S. | ||
Very recently, the same group reported another interesting serinol-modified 1,8-naphthalimide fluorescent probe, Nap-18, for the selective detection of H2S in human serum and in samples from hypertensive patients (Fig. 14).71 The probe incorporates an azide group as the H2S-reactive site, which undergoes reduction to an amine upon exposure to H2S, giving distinct optical responses. UV-visible spectroscopy exhibited the disappearance of the characteristic absorption band of the azide precursor and the emergence of new features consistent with the formation of the reduced amino-naphthalimide derivative. Fluorescence spectroscopy revealed a pronounced enhancement in emission intensity at λ = 520 nm upon treatment with Na2S, attributed to the ICT transition. The fluorescence intensity increased with H2S concentration (5–20 μM) and reached a plateau after about 20 minutes of incubation at pH 7.4, within physiological range. The limit of detection was calculated to be 0.16 μM. The probe exhibited excellent selectivity toward H2S over other biologically relevant thiols (Cys, GSH) and oxidative species. Quantitative analysis of human serum using Nap-18 yielded an H2S concentration of 17.98 μM, which correlated well with the value obtained by the standard methylene-blue UV-vis method (17.32 μM). Furthermore, significantly lower H2S levels were detected in hypertensive patient samples, indicating the probe's potential for biomedical and clinical diagnostics.
The design of H2S sensing probes has garnered significant interest, even within the computational domain. Haixiang He and colleagues employed density functional theory (DFT) and time-dependent DFT (TD-DFT) calculations, incorporating Grimme's D3 dispersion corrections and solvent modeling, to clarify the mechanisms of excited-state relaxation and fluorescence quenching of two 1,8-naphthalimide-based fluorescent probes (Nap-19 and Nap-20) for H2S detection (Fig. 15).72 The authors revealed that fluorescence quenching arises from a strong coupling between the locally excited (LE) state and a closely lying dark charge-transfer (CT) state, with the system favoring non-radiative decay via a minimum energy conical intersection (MECI) leading to a distorted naphthalimide structure. Structural analyses demonstrate how long-chain N-substituents influence geometrical distortions and electronic distributions that facilitate the decay pathway. Upon reacting with H2S, the resulting product molecules exhibit an increased energy gap between the LE and the CT states, losing the accessible MECI, thereby favoring radiative relaxation and producing strong fluorescence, in line with experimental results. The study further validated theoretical absorption and emission spectra alongside quantum yield calculations against experimental data, reinforcing the mechanistic insights. This work highlighted the critical role of MECIs in modulating probe fluorescence and underscores how computational insights can guide the rational design of enhanced fluorescent probes for biological gas sensing, offering a valuable framework for interpreting photophysical behaviours in related systems.
2.3. H2S sensing by deprotection – thiolysis
Naphthalimide derivatives are well known for their strong fluorescence, which arises from an internal charge transfer (ICT) transition between an electron-donating substituent (such as an amine or hydroxyl group) on the naphthalene ring and the imide moiety. When the electron-donating ability of such substituents is suppressed through protective modification, the ICT process is attenuated or completely blocked. However, upon H2S-triggered deprotection, the free hydroxyl/amine group is restored, thereby re-establishing the ICT pathway and producing a significant fluorescence enhancement. This thiolysis-based strategy has been applied in the design of several H2S-responsive naphthalimide probes.In 2019, Hongmei and co-workers were the first to report naphthalimide sensors that explicitly utilized this protection–deprotection approach for selective H2S detection.73 They developed two 1,8-naphthalimide-based fluorescent probes (Nap-21 and Nap-22) for the selective detection of H2S (Fig. 16). By combining 4-hydroxy-1,8-naphthalimide as the fluorophore with 2,4-dinitrophenyl ether as the H2S-responsive site, they achieved a photoinduced electron transfer (PET)-based sensing system. Upon addition of H2S, the probes displayed a distinct color change from colorless to yellow, accompanied by a large red shift in absorption (>100 nm) and more than 30-fold enhancement of yellow fluorescence emission near λ = 548–549 nm, enabling both fluorometric and naked-eye detection. Notably, the dual-site probe Nap-22 provided a broader linear detection range (0–40 μM) compared to the single-site analogue Nap-21 (0–30 μM), enabling more reliable quantification at higher H2S levels. The probes demonstrated good selectivity against common interfering species and maintained functionality across biologically relevant pH ranges (5–8). Mechanistic investigations, supported by NMR and TD-DFT calculations, confirmed that thiolysis of the 2,4-dinitrophenyl ether moiety triggers the fluorescence turn-on response. Importantly, practical tests in tap water and rainwater validated their potential for environmental monitoring applications.
 | ||
| Fig. 16 Structure of Nap-21, Nap-22, and Nap-23 and their hydroxyl product formed after thiolysis by H2S. | ||
Based on the same strategy, S. Hu et al. designed a novel two-photon fluorescent probe (Nap-23) from 4-hydroxy-1,8-naphthalimide, using 2-fluoro-5-nitrobenzoic ester as a protection group (Fig. 16).74 They have explored the ability of the designed two-photon probe for quantitatively detecting hydrogen polysulfides (H2Sn, n > 1), and it exhibited selectivity towards H2Sn over other reactive sulfur species (RSS), especially H2S and biothiols. The probe is based on a 4-hydroxy-1,8-naphthalimide fluorophore in a donor–π–acceptor framework, with fluorescence initially quenched by a 2-fluoro-5-nitrobenzoic ester group. Upon reaction with H2Sn, thiolysis removes this protective group, restoring the ICT process and producing a strong “turn-on” fluorescence response. Nap-23 exhibited an over 80-fold fluorescence enhancement at 550 nm, and a detection limit as low as 33 nM. A key strength of this study is the application of two-photon excitation (λ = 820 nm), which provided deeper tissue penetration and high-resolution imaging compared to traditional one-photon probes. The probe was successfully used for fluorescence imaging of H2Sn in live HeLa cells, demonstrating low cytotoxicity and good cell permeability. The fluorescence was stable across a biologically relevant pH range (4–8), further supporting its suitability for cellular applications.
Xu et al. developed a PET-regulated naphthalimide-based turn-on fluorescent probe (Nap-24) for the detection of H2S and biothiols, in which a 2,4-dinitrobenzenesulfonyl (DNBS) group functions simultaneously as the recognition unit and a strong electron acceptor (Fig. 17A).75 In the absence of target analytes, the excited-state fluorescence of the naphthalimide–benzothiazole fluorophore is efficiently quenched through PET to the DNBS moiety, resulting in an almost non-emissive background. Upon exposure to H2S, cleavage of the DNBS group effectively inhibits the PET process and restores the intrinsic emission of the naphthalimide core, giving rise to a pronounced fluorescence turn-on centered at approximately λ = 551 nm, along with intensified absorption bands around λ = 385 and 470 nm (Fig. 17B). Probe Nap-24 exhibits fast response kinetics (≤200 s), high sensitivity with a submicromolar detection limit for H2S, and good selectivity over common amino acids and reactive species across a physiologically relevant pH window (Fig. 17C–E). Beyond solution-phase sensing, the probe shows excellent biocompatibility and robust performance in complex biological environments, enabling both one- and two-photon fluorescence imaging of H2S/thiols in living HeLa cells, deep mouse liver tissues, and zebrafish. The large two-photon absorption cross section and effective tissue penetration underscore the potential of PET-based naphthalimide probes for in vivo visualization of reactive sulfur species, although discrimination between H2S and endogenous thiols remains an inherent limitation of DNBS-based sensing strategies.
 | ||
| Fig. 17 (A) Structure of Nap-24 and its product formed after reaction with H2S. (B) The proposed sensing mechanism for the detection of H2S. (C) Time (0 to 200 s) vs. fluorescence intensity (I551) curves of probe Nap-24 in the presence of various thiols and H2S in aqueous solution. (D) The fluorescence intensities (I551) of Nap-24 mixed with various interferents in aqueous solution. (E) The ultraviolet light image of Nap-24 with the detection target and interfering amino acids. Reproduced with permission from ref. 75. Copyright 2019, Royal Society of Chemistry. | ||
Liu and co-workers later reported a 1,8-naphthalimide–ebselen fluorescent probe (Nap-25) for highly selective and sensitive detection of H2S in both biological and environmental samples.76 The probe displayed a rapid turn-on fluorescence response with a high quantum yield (Φ = 0.5932), a large Stokes shift of 125 nm, and excellent stability over a broad pH range (5–11), making it well-suited for physiological conditions. Mechanistic investigations using NMR titration and HRMS established that the sensing process involves H2S-mediated nucleophilic cleavage of the Se–N bond in the ebselen moiety, generating an electron-donating amino group at the 4-position of the 1,8-naphthalimide core (Fig. 18A). This transformation enhances intramolecular charge transfer (ICT), producing a pronounced fluorescence increase that directly links the molecular design to its sensing function. Probe Nap-25 demonstrated excellent selectivity against common ions, amino acids, and biomolecules, with competitive assays confirming that its response to H2S remains unaffected by high concentrations of potential interferents such as glutathione (GSH) (Fig. 18B). It featured a low detection limit of 0.129 μM and a fast response time (<90 s), making it suitable for real-time monitoring. In biological studies, Nap-25 exhibited low cytotoxicity in GBC-SD cells and enabled clear confocal imaging of exogenous H2S, with fluorescence intensity increasing proportionally to H2S concentration (Fig. 18C–F). Beyond cellular imaging, Nap-25 was also validated for environmental monitoring, where quantitative detection in tap, river, and seawater samples yielded high recovery rates (92–102%), highlighting its robustness and broad applicability.
 | ||
| Fig. 18 (A) The proposed reactivity-based sensing mechanism for the binding of Nap-25 with H2S. (B) The extent of changes in fluorescence emission for Nap-25 after adding various analytes. (C) Fluorescence spectra of probe Nap-25 (10 μM) in a mixture of PBS buffer (pH = 7.4) and DMSO (10%) upon the gradual mixing of H2S with different concentrations. (D–F) Fluorescence imaging of cells incubated with Nap-25 and varied concentrations of Na2S. From (D) to (F), the concentrations of Na2S are 0, 50, and 100 μM, respectively. Reproduced with permission from ref. 76. Copyright 2022, Elsevier. | ||
In 2024, Huang et al. reported a detailed theoretical study on three ratiometric 1,8-naphthalimide fluorescent probes (Nap-26, Nap-27, and Nap-28) for H2S detection using DFT and TDDFT methods (see Fig. 19A).77 The theoretical calculations showed that while substituent modifications have minimal effect on the core 1,8-naphthalimide skeleton, they significantly influence local bond angles, dihedral angles, and electronic properties, leading to redshifts in absorption and emission spectra when electron-withdrawing strength at C4 or electron-donating ability at the imide nitrogen increases. Importantly, the study suggested that for one probe (Nap-26), the true sensing species is a deprotonated product rather than the neutral hydroxylated form proposed experimentally, offering new mechanistic insight. Frontier molecular orbital analysis confirmed the presence of intramolecular charge transfer (ICT) in both probes and their products, while the energy gap (ΔEH–L) analysis explained substituent-dependent spectral shifts (Fig. 19B). By providing a molecular-level rationale that aligns well with experimental observations, this work bridges theory and experiment, offering valuable design principles for developing next-generation ratiometric 1,8-naphthalimide probes with enhanced performance in H2S sensing.
 | ||
| Fig. 19 (A) Molecular structures of probes Nap-26, Nap-27, and Nap-28. (B) Frontier molecular orbital distributions and corresponding energies of probe Nap-26 (labelled as LA) and its product (labelled as LA-pro-O−). Reproduced with permission from ref. 77. Copyright 2025, Springer. | ||
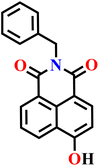
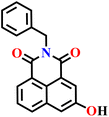
Very recently, Dvorak and co-workers investigated hydroxy-1,8-naphthalimide derivatives (Nap-29 to Nap-32) as scaffolds for reaction-based fluorescent probes, leveraging their strong photo-acidity and excited-state proton transfer (ESPT) behaviour to achieve red-shifted emission (Fig. 20A).78 These probes were carefully designed to assess the influence of hydroxyl positional isomerism and electrophilic recognition motifs on H2S sensing. 2,4-Dinitrobenzenesulfonyl ester (DNBS) and 2,4-dinitrobenzene ether (DNBE) were employed as effective recognition motifs. Nap-29 with a DNBS group displayed dual “turn-on” fluorescence bands at λ = 455 and 599 nm upon H2S-triggered nucleophilic aromatic substitution reaction, with rapid response kinetics (k ≈ 8.2 × 102 M−1 s−1) and a detection limit of 3.34 μM (Fig. 20B and C). Comparative studies showed that Nap-31 exhibited higher sensitivity than Nap-29, owing to the greater stability of its 4-naphtholate intermediate, although it did not display dual ESPT emission. Nap-30 served as a mechanistic control, confirming that nucleophilic substitution at the naphthalene ring requires unnaturally high H2S concentrations, while Nap-32 showed lower sensitivity but improved selectivity over biothiols such as cysteine and glutathione. Importantly, confocal imaging in MDA-MB-231 cells validated the cellular applicability of Nap-29, with significant fluorescence enhancement correlating to intracellular H2S levels and minimal cytotoxicity (Fig. 20D). This systematic structure–function analysis demonstrates that hydroxyl positioning and recognition-group chemistry are key determinants of ESPT efficiency, fluorescence output, sensitivity, and selectivity, providing guiding principles for designing next-generation H2S probes with optimized red-shifted emission, reactivity, and biological compatibility.
 | ||
Fig. 20 (A) Molecular structures of probes Nap-29, Nap-30, Nap-31, and Nap-32. (B) Fluorescence emission spectra of Nap-29 (10 μM) upon titration with increasing concentrations of NaHS (0–120 μM) in DMSO-phosphate buffer (0.01 M) at pH 7.4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) at room temperature (λex = 351 nm) (inset: color changes observed before and after the addition of NaHS). (C) Fluorescence response of Nap-29 (10 μM) upon addition of various analytes. (D) Confocal images of MDA-MB-231: (top row) untreated control cells, (middle row) cells treated with only 15 μM Nap-29 probe, (bottom row) cells pretreated with 300 μM NaHS followed by treatment with 15 μM Nap-29 probe. Reproduced with permission from ref. 78. Copyright 2025, American Chemical Society. 7, v/v) at room temperature (λex = 351 nm) (inset: color changes observed before and after the addition of NaHS). (C) Fluorescence response of Nap-29 (10 μM) upon addition of various analytes. (D) Confocal images of MDA-MB-231: (top row) untreated control cells, (middle row) cells treated with only 15 μM Nap-29 probe, (bottom row) cells pretreated with 300 μM NaHS followed by treatment with 15 μM Nap-29 probe. Reproduced with permission from ref. 78. Copyright 2025, American Chemical Society. | ||
3. Conclusions and outlook
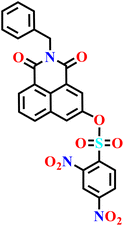
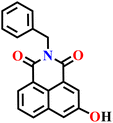
In this review, we have provided a comprehensive overview of recent progress in the design and development of reactivity-based 1,8-naphthalimide fluorescent probes for H2S detection. These systems employ a variety of sensing strategies-including nitro/azide reduction, thiolysis-mediated deprotection, nucleophilic substitution, and excited-state proton transfer (ESPT), each offering distinct mechanistic advantages. Table 1 summarizes the key sensing properties of the reported 1,8-naphthalimide-based chemosensors. Throughout the review, we emphasized how specific structural modifications influence sensing pathways, selectivity, sensitivity, and functional performance in biological and environmental settings. Collectively, these insights underscore the versatility of the 1,8-naphthalimide scaffold as a robust platform for constructing high-performance H2S probes. By consolidating current developments and highlighting structure–function relationships, this review aims to serve as a valuable resource for researchers in chemical sensing, bioimaging, and analytical chemistry.| Probe | Product | Medium | LoD | Reaction time | Ref. |
|---|---|---|---|---|---|
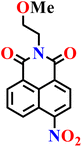
|
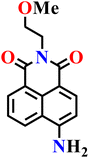
|
Aqueous buffer (50 mM PIPES, 100 mM KCl, pH 7.4) | 5–10 μM | 90 min | 45 |
| Nap-1 | |||||
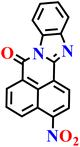
|
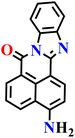
|
DMSO–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) medium 1) medium |
μM | 20 min | 57 |
| Nap-2 | |||||
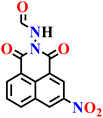
|
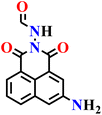
|
DMSO | 8.1 μM | NR | 58 |
| Nap-3 | |||||
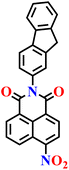
|
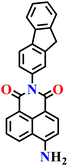
|
DMSO 0.5%; PBS 99.5% | 14 mM | NR | 59 |
| Nap-4 | |||||
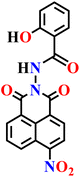
|
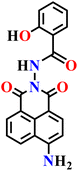
|
0.25% DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99.75% PBS 99.75% PBS |
12.33 nM | 20 h | 60 |
| Nap-5 | |||||
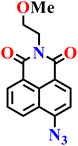
|
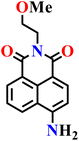
|
Aqueous buffer (50 mM PIPES, 100 mM KCl, pH 7.4) | 1–5 μM | 45 min | 45 |
| Nap-6 | |||||
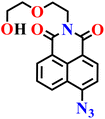
|
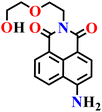
|
Aqueous DMSO solution (H2O–DMSO, pH 7.4) | 0.37 μM and 1.4 ppb in real samples | 15 min | 61 |
| Nap-7 | |||||
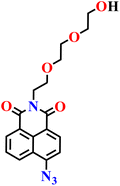
|
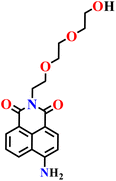
|
PBS buffer (10 μM, pH 7.4) | Less than 0.3 μM | 30 min | 62 |
| Nap-8 | |||||
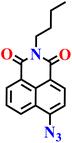
|
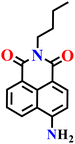
|
PBS buffer (10 μM, pH 7.4) | μM | 30 min | 62 |
| Nap-9 | |||||
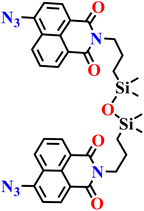
|
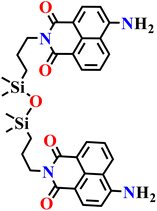
|
Aqueous solution (25 μM PBS buffer, pH = 7.4, containing 10% ethanol) | 0.57 μM | 12 min | 63 |
| Nap-10 | |||||
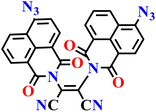
|
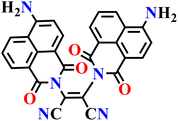
|
PBS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (1 DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4) 1, v/v, pH 7.4) |
1.5 μM | 2 s | 64 |
| Nap-11 | |||||
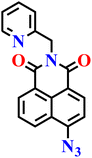
|
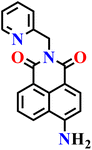
|
Acetonitrile/water (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v) 2 v/v) |
1.2 μM. | ∼8–10 s | 65 |
| Nap-12 | |||||
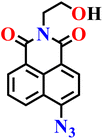
|
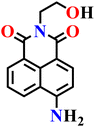
|
DMSO | 1 × 10−7 M | 10 s | 66 |
| Nap-13 | |||||
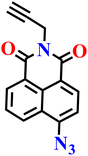
|
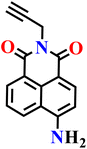
|
CH3CN/PBS buffer (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 1 v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20 mmol L−1, pH 7.4) 1, 20 mmol L−1, pH 7.4) |
1.18 μM | 3 min | 67 |
| Nap-14 | |||||
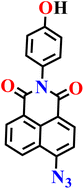
|
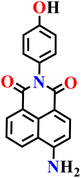
|
DMF/PBS solution (v/v, 7/3, pH 7.4, 20.0 mM) | 0.085 μM | 3 min | 68 |
| Nap-15 | |||||
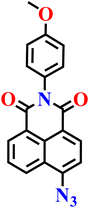
|
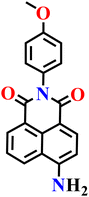
|
PBS buffer | 17.4 nM | NR | 69 |
| Nap-16 | |||||
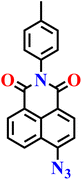
|
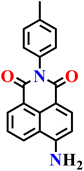
|
PBS buffer (pH 5.8–8.0) | 0.085 μM | 15 min | 70 |
| Nap-17 | |||||
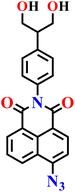
|
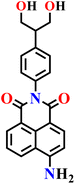
|
Ethanol/water | 0.16 μmol L−1 | 30 min | 71 |
| Nap-18 | |||||
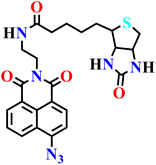
|
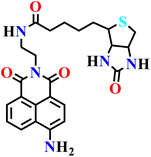
|
NR | NR | NR | 72 |
| Nap-19 | |||||
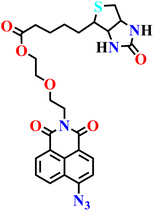
|
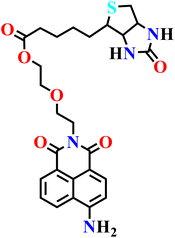
|
NR | NR | NR | 72 |
| Nap-20 | |||||
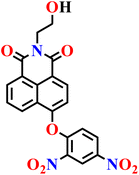
|
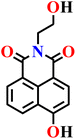
|
PBS/EtOH solution | 1.54 μmol L−1 | 120 min | 73 |
| Nap-21 | |||||
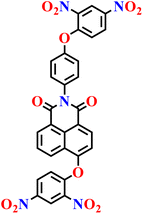
|
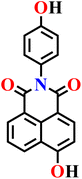
|
PBS/EtOH solution | 1.40 μmol L−1 | 120 min | 73 |
| Nap-22 | |||||
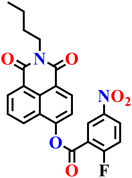
|
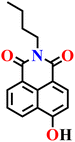
|
10 mM phosphate buffer solution (containing 1% DMSO as a co-solvent) | 33 nM. | 5 min | 74 |
| Nap-23 | |||||
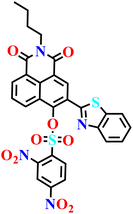
|
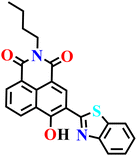
|
Aqueous solution (25 mM PBS, pH 7.4, mixed with 20% EtOH) | 22.2 μM | 2 min | 75 |
| Nap-24 | |||||

|
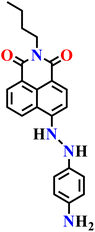
|
Mixture of PBS buffer (pH 7.4) and DMSO (10%) | 0.129 μM | 90 s | 76 |
| Nap-25 | |||||
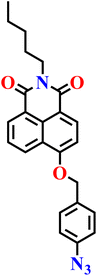
|
NR | NR | NR | NR | 77 |
| Nap-26 | |||||
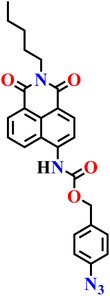
|
NR | NR | NR | NR | 77 |
| Nap-27 | |||||
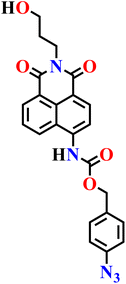
|
NR | NR | NR | NR | 77 |
| Nap-28 | |||||
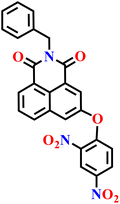
|
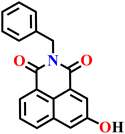
|
DMSO–phosphate buffer (0.01 M) at pH 7.4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) 7, v/v) |
3.34 μM | 15 min | 78 |
| Nap-29 | |||||
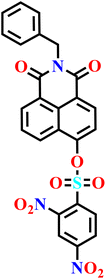
|
|
DMSO–phosphate buffer (0.01 M) at pH 7.4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) 7, v/v) |
μM | NR | 78 |
| Nap-30 | |||||
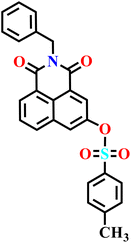
|
|
DMSO–phosphate buffer (0.01 M) at pH 7.4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) 7, v/v) |
μM | NR | 78 |
| Nap-31 | |||||
|
|
DMSO | μM | 10 min | 78 |
| Nap-32 |
Despite significant advances, several challenges remain before these probes can be fully translated into practical applications. Most reported systems rely on simple turn-on fluorescence, which, although sensitive, can suffer from background interference. Greater emphasis on ratiometric probe design, providing internal self-calibration, greatly enhances quantitative reliability. Furthermore, while ICT modulation dominates current strategies, expanding into dual-mode (electrochemical–optical) and ESPT- or FRET-based platforms could improve selectivity and performance in complex media. Selectivity remains a persistent issue, especially in the presence of abundant endogenous biothiols like GSH and cysteine, which can compete with H2S. Improved recognition motifs and strategic incorporation of electron-withdrawing groups may help minimize such cross-reactivity.
Reaction kinetics also warrant further optimization. Many existing probes respond slowly due to limited reactivity at aromatic trigger sites. Incorporating more reactive motifs—such as aliphatic amines, selenium-based groups, or strained electrophiles—could substantially accelerate response times. Although several probes now reach nanomolar detection limits, their stability and performance under physiologically relevant conditions must be strengthened for reliable in vivo imaging. Progressing from cell-level demonstrations to tissue and whole-animal imaging will require probes with enhanced aqueous solubility, high photostability, and compatibility with near-infrared or two-photon excitation for deeper tissue penetration and minimal autofluorescence.
Among the different probe types, thiolysis-based systems generally offer the fastest response but are more prone to interference from abundant biothiols, while reduction-based probes provide higher specificity toward H2S but often respond more slowly. Nucleophilic substitution probes are synthetically simple yet more susceptible to false signals in thiol-rich environments. In contrast, ESPT- and FRET-based designs offer more stable or ratiometric outputs, making them better suited for accurate imaging in complex biological samples. Overall, each sensing strategy presents distinct strengths and limitations, underscoring the importance of selecting or designing probes based on the specific demands of the intended application.
In conclusion, 1,8-naphthalimide-based fluorescent probes represent a powerful and continually evolving platform for H2S sensing. Continued innovation in probe design, targeting improved selectivity, faster kinetics, and advanced imaging capabilities, is expected to yield next-generation systems capable of real-time H2S monitoring across diverse biological and environmental contexts. Looking forward, such probes hold significant promise for applications in disease diagnostics, redox biology, therapeutic monitoring, environmental pollutant analysis, and imaging-guided studies of sulfur-mediated signaling pathways. These opportunities highlight both the broad impact and the future potential of the developments summarized in this review.
Author contributions
M. K. Noushija: conceptualization, software, resources, visualization, funding acquisition, writing – original draft, A. V. Krishna: software, resources, visualization, writing – original draft. S. Shanmugaraju: conceptualization, software, resources, visualization, project administration, supervision, investigation, funding acquisition, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
No new data were generated or analysed as part of this review.Acknowledgements
We gratefully acknowledge financial support from the Anusandhan National Research Foundation, India (EMEQ research grant EEQ/2023/000386 and CRG research grant CRG/2023/006085 awarded to SS), and from the Department of Science and Technology, Government of India (India-Japan Science and Technology Cooperation research grant DST/ICD/JSPS/2024/698 to SS).Notes and references
- R. Wang, Trends Biochem. Sci., 2014, 39, 227–232 CrossRef CAS PubMed.
- C. Mancuso, P. Navarra and P. Preziosi, J. Neurochem., 2010, 113, 563–575 CrossRef CAS PubMed.
- O. Kabil and R. Banerjee, J. Biol. Chem., 2010, 285, 21903–21907 CrossRef CAS PubMed.
- K. Qu, S. W. Lee, J. S. Bian, C.-M. Low and P.-H. Wong, Neurochem. Int., 2008, 52, 155–165 CrossRef CAS PubMed.
- C. Chen, H. Xin and Y. Zhu, Acta Pharmacol. Sin., 2007, 28, 1709–1716 CrossRef CAS PubMed.
- W. A. Pryor, Am. J. Physiol., 2006, 291, R491–R511 CAS.
- D. R. Lide and H. P. R. Frederikse, Handbook of Chemistry and Physics, CRC Press, Boca Raton, FL, 76th edn, 1995 Search PubMed.
- G. Yang, L. Wu, B. Jiang, W. Yang, J. Qi, K. Cao, Q. Meng, A. K. Mustafa, W. Mu and S. Zhang, Science, 2008, 322, 587–590 CrossRef CAS PubMed.
- N. Shibuya, M. Tanaka, M. Yoshida, Y. Ogasawara, T. Togawa, K. Ishii and H. Kimura, Antioxid. Redox Signaling, 2009, 11, 703–714 CrossRef CAS PubMed.
- R. Hosoki, N. Matsuki and H. Kimura, Biochem. Biophys. Res. Commun., 1997, 237, 527–531 CrossRef CAS PubMed.
- K. Abe and H. Kimura, J. Neurosci., 1996, 16, 1066–1071 CrossRef CAS PubMed.
- L.-F. Hu, M. Lu, P. T. Hon Wong and J.-S. Bian, Antioxid. Redox Signaling, 2011, 15, 405–419 CrossRef CAS PubMed.
- N. S. Lawrence, J. Davis and R. G. Compton, Talanta, 2000, 52, 771–784 CrossRef CAS PubMed.
- M. M. Gadalla and S. H. Snyder, J. Neurochem., 2010, 113, 14–26 CrossRef CAS PubMed.
- M. Lee, C. Schwab, S. Yu, E. McGeer and P. L. McGeer, Neurobiol. Aging, 2009, 30, 1523–1534 CrossRef CAS PubMed.
- E. Jw, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 15560–15565 Search PubMed.
- K. Qu, C. P. L. H. Chen, B. Halliwell, P. K. Moore and P. T.-H. Wong, Stroke, 2006, 37, 889–893 CrossRef CAS PubMed.
- A. Sivarajah, M. C. McDonald and C. Thiemermann, Shock, 2006, 26, 154–161 CrossRef CAS PubMed.
- A. Ichinohe, T. Kanaumi, S. Takashima, Y. Enokido, Y. Nagai and H. Kimura, Biochem. Biophys. Res. Commun., 2005, 338, 1547–1550 CrossRef CAS PubMed.
- H. Yan, J. Du and C. Tang, Biochem. Biophys. Res. Commun., 2004, 313, 22–27 CrossRef CAS PubMed.
- P. Kamoun, M. Belardinelli, A. Chabli, K. Lallouchi and B. Chadefaux-Vekemans, Am. J. Med. Genet., Part A, 2003, 116, 310–311 CrossRef PubMed.
- K. Eto, T. Asada, K. Arima, T. Makifuchi and H. Kimura, Biochem. Biophys. Res. Commun., 2002, 293, 1485–1488 CrossRef CAS PubMed.
- J. E. Doeller, T. S. Isbell, G. Benavides, J. Koenitzer, H. Patel, R. P. Patel, J. R. Lancaster Jr, V. M. Darley-Usmar and D. W. Kraus, Anal. Biochem., 2005, 341, 40–51 CrossRef CAS PubMed.
- T. Ubuka, J. Chromatogr., B, 2002, 781, 227–249 Search PubMed.
- N. S. Lawrence, J. Davis, L. Jiang, T. G. J. Jones, S. N. Davies and R. G. Compton, Electroanalysis, 2000, 12, 1453–1460 CrossRef CAS.
- J. Radford-Knoery and G. A. Cutter, Anal. Chem., 1993, 65, 976–982 CrossRef CAS.
- J. C. Savage and D. H. Gould, J. Chromatogr. B: Biomed. Sci. Appl., 1990, 526, 540–545 CrossRef CAS PubMed.
- G. K. Kolluru, X. Shen, S. C. Bir and C. G. Kevil, Nitric Oxide, 2013, 35, 5–20 CrossRef CAS PubMed.
- L. D. Lavis and R. T. Raines, ACS Chem. Biol., 2008, 3, 142–155 CrossRef CAS PubMed.
- E. L. Que, D. W. Domaille and C. J. Chang, Chem. Rev., 2008, 108, 1517–1549 CrossRef CAS PubMed.
- P. Wardman, Free Radical Biol. Med., 2007, 43, 995–1022 CrossRef CAS PubMed.
- J. Pan, J. Xu, Y. Zhang, L. Wang, C. Qin, L. Zeng and Y. Zhang, Spectrochim. Acta, Part A, 2016, 168, 132–138 CrossRef CAS PubMed.
- J. Hong, W. Feng and G. Feng, Sens. Actuators, B, 2018, 262, 837–844 CrossRef CAS.
- W. Luo, H. Xue, J. Ma, L. Wang and W. Liu, Anal. Chim. Acta, 2019, 1077, 273–280 CrossRef CAS PubMed.
- K. Zhong, L. Chen, X. Yan, Y. Tang, S. Hou, X. Li and L. Tang, Dyes Pigm., 2020, 182, 108656 Search PubMed.
- H. Zhang, X. Yue, W. Li, W. Chen, Y. Wang, X. Li, Y. Ye and X. Song, Sens. Actuators, B, 2021, 331, 129394 Search PubMed.
- K. Liu, C. Liu, H. Shang, M. Ren and W. Lin, Sens. Actuators, B, 2018, 256, 342–350 Search PubMed.
- W. Chen, S. Chen, B. Zhou, H. Wang, X. Song and H. Zhang, Dyes Pigm., 2015, 113, 596–601 Search PubMed.
- W. Xuan, C. Sheng, Y. Cao, W. He and W. Wang, Angew. Chem., Int. Ed., 2012, 51, 2282–2284 Search PubMed.
- W.-X. Wang, Z.-Q. Wang, Z.-K. Tan, G.-J. Mao, D.-H. Chen and C.-Y. Li, Analyst, 2022, 147, 2712–2717 Search PubMed.
- X.-G. Chen, Y. Mei, H. Li and Q.-H. Song, Sens. Actuators, B, 2022, 354, 131202 CrossRef CAS.
- S.-J. Li, Y.-F. Li, H.-W. Liu, D.-Y. Zhou, W.-L. Jiang, J. Ou-Yang and C.-Y. Li, Anal. Chem., 2018, 90, 9418–9425 Search PubMed.
- R. M. Duke, E. B. Veale, F. M. Pfeffer, P. E. Kruger and T. Gunnlaugsson, Chem. Soc. Rev., 2010, 39, 3936–3953 Search PubMed.
- W. W. Stewart, Nature, 1981, 292, 17–21 Search PubMed.
- L. A. Montoya and M. D. Pluth, Chem. Commun., 2012, 48, 4767–4769 RSC.
- Z. Xu, L. Xu, J. Zhou, Y. Xu, W. Zhu and X. Qian, Chem. Commun., 2012, 48, 10871–10873 RSC.
- X. Cao, W. Lin, K. Zheng and L. He, Chem. Commun., 2012, 48, 10529–10531 RSC.
- C. Liu, J. Pan, S. Li, Y. Zhao, L. Y. Wu, C. E. Berkman, A. R. Whorton and M. Xian, Angew. Chem., Int. Ed., 2011, 50, 10327 CrossRef CAS PubMed.
- X. Wu, H. Li, Y. Kan and B. Yin, Dalton Trans., 2013, 42, 16302–16310 Search PubMed.
- C. Kar and G. Das, J. Photochem. Photobiol., A, 2013, 251, 128–133 CrossRef CAS.
- X.-F. Yang, L. Wang, H. Xu and M. Zhao, Anal. Chim. Acta, 2009, 631, 91–95 Search PubMed.
- M. G. Choi, S. Cha, H. Lee, H. L. Jeon and S.-K. Chang, Chem. Commun., 2009, 7390–7392 Search PubMed.
- G. Kim, E. Jang, A. M. Page, T. Ding, K. A. Carlson and H. Cao, RSC Adv., 2016, 6, 95920–95924 RSC.
- T. Annaka, N. Nakata and A. Ishii, New J. Chem., 2019, 43, 11643–11652 RSC.
- Q. Wu, F. Huo, J. Wang and C. Yin, Spectrochim. Acta, Part A, 2020, 238, 118437 CrossRef CAS PubMed.
- X. Shen, C. B. Pattillo, S. Pardue, S. C. Bir, R. Wang and C. G. Kevil, Free Radical Biol. Med., 2011, 50, 1021–1031 CrossRef CAS PubMed.
- A. Bamesberger, G. Kim, J. Woo and H. Cao, J. Fluoresc., 2015, 25, 25–29 CrossRef CAS PubMed.
- S. Naha, S. P. Wu and S. Velmathi, RSC Adv., 2020, 10, 8751–8759 RSC.
- M. Ranjana, M. S. Shrilaxmi, A. Bera, D. Sunil, Y. N. Sudhakar, D. Upadhya, S. G. Dastidar, S. R. Vennapusa and S. D. Kulkarni, Microchem. J., 2025, 215, 114060 CrossRef CAS.
- M. Ranjana, N. N. Kashyap, D. Sunil, A. Bera, P. K. Mitra, P. P. Yegneswaran, Y. N. Sudhakar, D. Upadhya, S. D. Kulkarni and S. R. Vennapusa, Analyst, 2025, 150, 5245 RSC.
- Y. Guo, T. Zeng, G. Shi, Y. Cai and R. Xie, RSC Adv., 2014, 4, 33626–33628 RSC.
- S. A. Choi, C. S. Park, O. S. Kwon, H. K. Giong, J. S. Lee, T. H. Ha and C. S. Lee, Sci. Rep., 2016, 6, 1–10 Search PubMed.
- X. Wang, Y. Zuo, Y. Zhang, T. Yanga and W. Lin, Anal. Methods, 2020, 12, 1064–1069 RSC.
- D. Jothi, S. Munusamy and S. K. Iyer, J. Photochem. Photobiol., A, 2021, 420, 2–9 CrossRef.
- D. Jothi and S. K. Iyer, J. Photochem. Photobiol., A, 2022, 427, 113802 CrossRef CAS.
- Y. Yan, S. Zhu, Z. Chen and Y. Ji, J. Appl. Spectrosc., 2022, 89, 191–200 CrossRef CAS.
- Z. Jihua, Z. Hao, L. Min, L. Jingjiang, L. Yuan, Q. Zhengjun and W. Xicun, Chin. J. Org. Chem., 2020, 40, 1043–1049 CrossRef.
- J. Zhu, C. Miao and X. Wang, J. Photochem. Photobiol., A, 2023, 440, 114659 CrossRef CAS.
- Y. Zhang and L. Zhang, Microchem. J., 2020, 159, 105394 CrossRef CAS.
- A. Dandić, M. Samardžić, M. Budetić, I. D. Panić, I. Drenjančević, N. Kolobarić, G. Mikle, B. Kovács and A. Széchenyi, J. Fluoresc., 2024, 35, 1–9 Search PubMed.
- A. Széchenyi, M. Budetić, M. Samardžić, M. Stanković, I. Drenjančević, N. Kolobarić, B. Kovács, B. Lemli, G. Mikle and A. Dandić, J. Fluoresc., 2025 DOI:10.1007/s10895-025-04582-7.
- A. Huang, D. Wu, W. Hao and H. He, Spectrochim. Acta, Part A, 2025, 338, 126202 Search PubMed.
- Y. Ma, J. Zhang and H. Qu, Chem. Res. Chin. Univ., 2019, 35, 5–11 CrossRef CAS.
- L. Zhou, G. Yuan and S. Hu, J. Appl. Spectrosc., 2020, 86, 1071–1076 CrossRef CAS.
- K. Xu, L. He, Y. Yanga and W. Lin, New J. Chem., 2019, 43, 2865–2869 RSC.
- C. Zhang, L. Zhang, Y. Li, Z. Ren, L. Li, Y. Zhang, Y. Li and C. Liu, J. Mol. Struct., 2021, 1250, 131777 CrossRef.
- A. Huang, Y. Zhou, Y. Liang, Q. Liu, W. Hao, Z. Xia, D. Wu and H. He, J. Fluoresc., 2024, 35, 1–8 Search PubMed.
- T. Dvorak, S. Cheku, L. Borer, H. Hernandez-Sandoval, K. A. Carlson and H. Cao, ACS Omega, 2025, 10, 31138–31146 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |