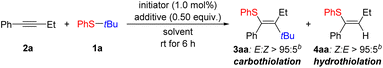Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Regio- and stereoselective tert-butylthiolation of internal alkynes with thioethers initiated and maintained by silylium-ion catalysis
Dáiríne M.
Morgan
 ,
Hendrik F. T.
Klare
,
Hendrik F. T.
Klare
 and
Martin
Oestreich
and
Martin
Oestreich
 *
*
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany. E-mail: martin.oestreich@tu-berlin.de
First published on 2nd January 2026
Abstract
A two-component protocol for the regio- and trans-selective addition of aryl tertiary alkyl (especially tert-butyl) thioethers across internal C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C triple bonds is reported. This carbothiolation is initiated by the catalytic formation of a silylated sulfonium ion as a tertiary carbenium ion (tert-butyl cation) source. Competing loss of a proton by β-elimination of that carbenium-ion intermediate and as such a potential hydrothiolation pathway are efficiently suppressed by the substoichiometric addition of an arylsilane as a “proton-into-silylium ion” generator. Through this, the silylium-ion activation is restored, thereby maintaining the carbothiolation pathway. The method enables the synthesis of sterically crowded, fully substituted aryl vinyl sulfides as well as the sulfoxides and sulfones.
C triple bonds is reported. This carbothiolation is initiated by the catalytic formation of a silylated sulfonium ion as a tertiary carbenium ion (tert-butyl cation) source. Competing loss of a proton by β-elimination of that carbenium-ion intermediate and as such a potential hydrothiolation pathway are efficiently suppressed by the substoichiometric addition of an arylsilane as a “proton-into-silylium ion” generator. Through this, the silylium-ion activation is restored, thereby maintaining the carbothiolation pathway. The method enables the synthesis of sterically crowded, fully substituted aryl vinyl sulfides as well as the sulfoxides and sulfones.
Introduction
Alkenes are widely used in the synthesis of complex organic molecules and are themselves commonly found in natural products and bioactive compounds.1 Despite their ubiquity, the formation of alkenes is non-trivial with the necessity of considering both stereo- and (depending on the method) regioselectivity factors. This is particularly true for the delicate challenging synthesis of highly substituted alkenes, this being C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds with three or even four carbon substitutents.2,3 One possible approach to the synthesis of sterically encumbered alkenes is the selective difunctionalization of alkynes.4,5 A wide range of functional groups and carbon substituents have been added across C
C double bonds with three or even four carbon substitutents.2,3 One possible approach to the synthesis of sterically encumbered alkenes is the selective difunctionalization of alkynes.4,5 A wide range of functional groups and carbon substituents have been added across C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C triple bonds by either three- or two-component reaction systems.6–12 Three-component reactions involve the use of a separate nucleophile and electrophile component for sequential addition to the alkyne unit.13,14 From an atom-economy perspective the more attractive option however is a two-component reaction in which both units for the addition to the alkyne come from a single reactant.4,15 A carbon substituent that has proven particularly challenging to install is a quaternary carbon atom emerging from a tertiary reactant. Although a number of examples have been published where addition of a tertiary carbon center to a terminal alkyne is achieved,16–22 examples involving the addition to an internal alkyne are more limited and usually involve activation of the reactants.23–27
C triple bonds by either three- or two-component reaction systems.6–12 Three-component reactions involve the use of a separate nucleophile and electrophile component for sequential addition to the alkyne unit.13,14 From an atom-economy perspective the more attractive option however is a two-component reaction in which both units for the addition to the alkyne come from a single reactant.4,15 A carbon substituent that has proven particularly challenging to install is a quaternary carbon atom emerging from a tertiary reactant. Although a number of examples have been published where addition of a tertiary carbon center to a terminal alkyne is achieved,16–22 examples involving the addition to an internal alkyne are more limited and usually involve activation of the reactants.23–27
Our group recently showed that S–Si bonds can be selectively added across an internal triple bond in a silylium-ion28 promoted reaction (Scheme 1A).15 This two-component difunctionalization provided a reliable access to fully substituted double bonds with two synthetic handles for further functional-group manipulation. Given the intermediacy of various cationic species, we asked ourselves whether that strategy would allow for the release and transfer of tertiary carbenium ions from thioethers (Scheme 1B). This would correspond to an intermolecular carbothiolation of alkynes. We are only aware of a single related example where Nakamura and co-workers disclosed an intramolecular gold-catalyzed carbothiolation by starting from an allylated thiophenyl derivative (Scheme 1C).29 Since then, a number of metal-catalyzed carbothiolation reactions of alkynes have been developed29–36 but, to the best of our knowledge, none of these involved simple tertiary alkyl groups.
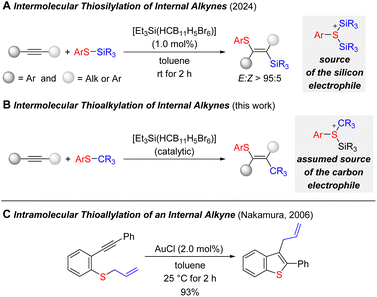 | ||
| Scheme 1 Silylium-ion-promoted thiosilylation and thioalkylation (carbothiolation) of internal alkynes. | ||
Results and discussion
We began our optimization using tert-butyl(phenyl)sulfane (1a) and but-1-yn-1-ylbenzene (2a) as model compounds (Table 1 and for full details see Tables S1–S5 in the SI). Beginning with the reaction conditions from the aforementioned thiosilylation,15 the desired carbothiolation product 3aa was obtained as a single stereoisomer in toluene with 1.0 mol% of [Me3Si(HCB11H5Br6)] in a 28% yield along with 18% yield of the hydrothiolation byproduct 4aa (entry 1). A survey of solvents revealed that (deuterated) benzene is an optimal choice, providing 3aa in a 43% yield with 17% of 4aa (entry 2). The reaction time could be reduced from 16 to 6 h with the yield maintained (entry 3). The equivalents of the starting material used played a significant role: Increasing the amount of the alkyne to two equivalents improved the yield of 3aa to 63% with 13% of 4aa (entry 4). This was likely due to the increased concentration of the alkyne accelerating the rate of the bimolecular process. If the volume of solvent used was increased, the overall yield reduced further pointing towards the importance of substrate concentration in this reaction (see Table S3 in the SI). A number of different initiators were then tested and, for example, the trityl salt [Ph3C][HCB11H5Br6] failed to initiate the reaction (entry 5) but the silylium salt [Et3Si(HCB11H5Br6)] improved the yield to 75% 3aa with 14% of 4aa (entry 6). We presumed that the hydrothiolation product 4aa was being generated by a proton released from the tertiary carbenium ion by β-elimination. We therefore added an arylsilane in order to trap this proton by proton-into-silylium ion interconversion.37–41 Ph4Si as an additive was almost completely insoluble, hence not bringing about any improvement (entry 7). Using 0.50 equiv. of Ph3SiH, it was possible to reduce the amount of 4aa to 5% while maintaining a 73% yield of the desired product 3aa (entry 8). Use of 1.0 equiv. of the alkyne led to a reduction in the yield of 3aa to 51% (entry 9).| Entry | Initiator | Solvent | 2a (equiv.) | Additive | Yield of 3aa (%)c | Yield of 4aa (%)c | Ratio of 3aa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4aad 4aad |
|---|---|---|---|---|---|---|---|
a All reactions were performed on a 0.20 mmol scale in a glovebox under an argon atmosphere in 0.5 mL of the indicated solvent.
b
E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratios estimated by 1H NMR spectroscopy of the crude reaction mixture.
c Yield determined by 1H NMR spectroscopy of the crude reaction mixture using CH2Br2 as an internal standard.
d
3aa Z ratios estimated by 1H NMR spectroscopy of the crude reaction mixture.
c Yield determined by 1H NMR spectroscopy of the crude reaction mixture using CH2Br2 as an internal standard.
d
3aa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4aa ratios determined by 1H NMR spectroscopy of the crude reaction mixture.
e Reaction time 16 h. n.r. = no reaction. 4aa ratios determined by 1H NMR spectroscopy of the crude reaction mixture.
e Reaction time 16 h. n.r. = no reaction.
|
|||||||
| 1e | [Me3Si(HCB11H5Br6)] | Toluene | 1.0 | 28 | 18 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 39 |
|
| 2e | [Me3Si(HCB11H5Br6)] | C6D6 | 1.0 | 43 | 17 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
|
| 3 | [Me3Si(HCB11H5Br6)] | C6D6 | 1.0 | 46 | 18 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
|
| 4 | [Me3Si(HCB11H5Br6)] | C6D6 | 2.0 | 63 | 13 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
|
| 5 | [Ph3C][HCB11H5Br6] | C6D6 | 2.0 | n.r. | n.r. | ||
| 6 | [Et3Si(HCB11H5Br6)] | C6D6 | 2.0 | 75 | 14 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
|
| 7 | [Et3Si(HCB11H5Br6)] | C6D6 | 2.0 | Ph4Si | 36 | 9 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 8 | [Et3Si(HCB11H5Br6)] | C6D6 | 2.0 | Ph3SiH | 73 | 5 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 9 | [Et3Si(HCB11H5Br6)] | C6D6 | 1.0 | Ph3SiH | 51 | 7 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
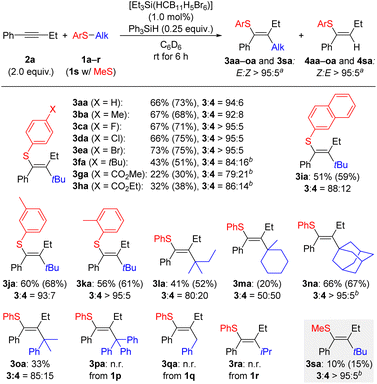
With the optimized conditions in hand, we turned to the substrate scope of the reaction. In many cases, separation of the product from unreacted Ph3SiH proved difficult, so the amount used was reduced from 0.50 to 0.25 equiv. We began gauging the substrate scope by varying the aryl ring on the thioether reagent 1 (Scheme 2). Methyl and halogen groups were well tolerated at the para position of the aryl group with only a small impact on the yield observed for 3ba–ea. When a bulkier group such as tert-butyl as in 1f was present, the activity of the reaction reduced significantly but a yield of 43% for 3fa could be obtained when the reaction temperature was increased to 60 °C. Although the yields remained low even at increased temperatures, products 3ga and 3ha bearing an ester group could also be obtained. The configuration of the double bond was assigned for 3ha by an nOe measurement (see Fig. S2 in the SI). A more sterically bulky β-naphthyl group in 1i resulted in a moderate yield for 3ia. Likewise, 1j and 1k with meta- and ortho-tolyl groups led to yields for 3ja and 3ka in the same range. Variation of the tertiary alkyl group was next looked at. When the tert-butyl group was replaced by a tert-amyl residue, a yield of 41% was obtained for 3la along with a substantial amount of the hydrothiolation product 4la. The hydrothiolation pathway became even more pronounced when a cyclic tertiary carbenium ion was present, resulting in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 3ma and 4ma. An adamantyl worked equally well as a tert-butyl group, leading to the formation of 3na in 66% yield with hardly any hydrothiolation byproduct. Any benzylic carbocation was either transferred sluggishly (1o → 3oa) or did not react as planned (trityl as in 1p and benzyl as in 1q). A secondary alkyl group as potentially released from isopropyl-substituted thioether 1r did not engage in the reaction. A dialkyl thioether such as 1s furnished the carbothiolation product 3sa only in poor yield (gray box).
1 ratio of 3ma and 4ma. An adamantyl worked equally well as a tert-butyl group, leading to the formation of 3na in 66% yield with hardly any hydrothiolation byproduct. Any benzylic carbocation was either transferred sluggishly (1o → 3oa) or did not react as planned (trityl as in 1p and benzyl as in 1q). A secondary alkyl group as potentially released from isopropyl-substituted thioether 1r did not engage in the reaction. A dialkyl thioether such as 1s furnished the carbothiolation product 3sa only in poor yield (gray box).
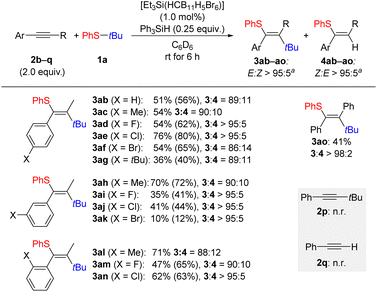
Following this, the aryl-substituted alkyne used was varied systematically (Scheme 3). Aliphatic alkynes were not compatible with this reaction with the aryl ring necessary for reaction initiation. Substitution at the para position of the aryl substituent in alkyne 2 was tolerated with moderate to good yields for 3ab–af. The exception to this was again a tert-butyl group which led to a reduction in yield to 36% for 3ag. Substitution at the meta position proved to be more challenging although a good yield was obtained for the tolyl-substituted derivative 3ah. The three halogenated products 3ai–ak were all obtained in low yields. In turn, substitution at the ortho position had little effect on the overall yield of the product 3al–an. It was possible to utilize diphenylacetylene (2o) in the reaction, and a moderate yield of 41% was found for the carbothiolation product 3ao. Conversely, no reaction occurred with an alkyne bearing a tert-butyl group as in 2p or with a terminal alkyne as for phenylacetylene (2q), respectively (gray box).
We believe that the mechanism of this carbothiolation reaction exhibits similarities to that of the silylium-ion-promoted thiosilylation of alkynes (see Scheme 1A).15 Interaction of the initiator [Et3Si(HCB11H5Br6)] with the aryl tert-butyl thioether was verified by a substituent exchange experiment (Scheme 4A). When thioether 1a was reacted with 20 mol% of the counteranion-stabilized silylium ion, the formation of the thiosilane 6 was detected by 29Si NMR spectroscopy, thereby suggesting the intermediacy of the silylsulfonium ion 5. Intermediate 5 is the starting point of the catalytic cycle (Scheme 4B). It transfers the tert-butyl cation onto the alkyne 2 to form the vinyl cation 7,42–48 which in turn reacts with excess thioether 1 to yield another sulfonium-ion intermediate 8. Although it is obvious that this sulfonium ion will release the tert-butyl cation to eventually liberate the carbothiolation product 3, its actual fate remains unclear. The role of sulfides 1 and 6 as carbenium-ion shuttles is a possibility but tert-butyl-substituted 1 to form bis-tert-butylated arylsulfonium ion 9 is unlikely for steric considerations and thiosilane 6 is only available at low concentration. Hence, we assume that the tert-butyl group is directly transferred from intermediate 8 to alkyne 2 thereby closing the catalytic cycle. The observation of the hydrothiolation product 4 lends further evidence for this pathway (Scheme 4C). Dissociation of any of the sulfonium ions 5, 8, or 9 gives access to a free tert-butyl cation 10, that suffers β-elimination by loss of a proton to give isobutene 11. That proton can be accepted by the alkyne 2 (or thioether 1 and silylated thiophenol 6) opening the door to the hydrothiolation channel. To suppress this side reaction, we decided to exploit the ability of [H(C6D6)][HCB11H5Br6] to convert arylsilanes into counteranion-stabilzed silylium ions by dearylation.37–41 That proton-into-silylium ion interconversion not only sequesters the strong Brønsted acid but also makes available [Ph2HSi(HCB11H5Br6)] (from Ph3SiH) for further sulfonium-formation. The arylsilane additive thereby enhances the overall performance of the catalysis.37,49 We also examined whether the carbothiolation is reversible but a scrambling experiment between product 3aa (Ar = Ph) and thioether 1b (Ar = 4-Tolyl) under the standard reaction conditions showed no exchange; the vinyl sulfide 3aa was recovered exclusively and a small amount of degradation was seen for 1b.
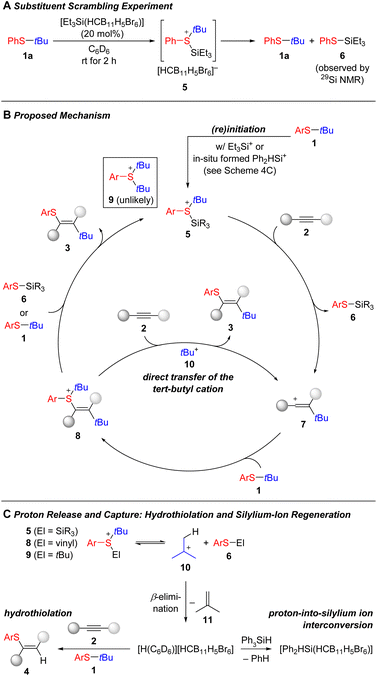 | ||
| Scheme 4 Discussion of the mechanism. The [HCB11H5Br6]− counterion is omitted for clarity in the catalytic cycle. | ||
To explore the utility of this reaction, we performed the model reaction on a 2.0-mmol scale, furnishing the vinyl sulfide 3aa in a slightly improved isolated yield of 72% (Scheme 5). In an effort to prime the carbon–sulfur bond in 3aa for further functional-group manipulation, we oxidized sulfur atom to the sulfoxide 12 with oxone in ethanol,50 and to the sulfone 13 with mCPBA.15 At this stage, attempts to engage any of these three vinyl components in a transition-metal-catalyzed cross-coupling have been unsuccessful in our hands.51–53
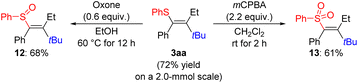 | ||
| Scheme 5 Oxidation of the fully substituted vinyl sulfide. Yields are isolated and refer to analytically pure material after flash chromatography on silica gel. | ||
Conclusions
We have here disclosed a new method for the regio- and trans-selective carbothiolation (thioalkylation) of internal alkynes that specifically enables the installation of a tert-butyl group at an alkene. A tert-butyl-substituted arylsulfide serves as the tert-butyl electrophile and at the same time as the sulfur nucleophile. This is made possible by initiation of the reaction with a counteranion-stabilized silylium ion to form a sulfonium ion as the actual carbenium-ion carrier. The reaction displays a good substrate scope with functional-group tolerance similar to that typically seen in other catalyses under superacidic conditions.Author contributions
D. M. M., H. F. T. K. and M. O. conceptualized this work. D. M. M. performed and analyzed the experiments. H. F. T. K. and M. O. supervised the research and acquired funding. All authors contributed to the writing and editing of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article has been included as part of the supplementary information (SI). Supplementary information: reaction optimizations, experimental procedures, full characterization data and copies of NMR spectra. See DOI: https://doi.org/10.1039/d5sc09722c.Acknowledgements
This research was supported by the Deutsche Forschungsgemeinschaft (Oe 249/30-1). M. O. is indebted to the Einstein Foundation Berlin for an endowed professorship.References
- P. Ertl and T. Schuhmann, J. Nat. Prod., 2019, 82, 1258–1263 CrossRef CAS.
- F. Buttard, J. Sharma and P. A. Champagne, Chem. Commun., 2021, 57, 4071–4088 RSC.
- A. B. Flynn and W. W. Ogilvie, Chem. Rev., 2007, 107, 4698–4745 CrossRef CAS PubMed.
- W. Liu and W. Kong, Org. Chem. Front., 2020, 7, 3941–3955 RSC.
- M. Piejko, J. Moran and D. Lebœuf, ACS Org. Inorg. Au, 2024, 4, 287–300 CrossRef CAS.
- S. R. Chemler and M. T. Bovino, ACS Catal., 2013, 3, 1076–1091 Search PubMed.
- S. Peng, X. Q. Xu, L. Tang, N.-D. Meng, L.-H. Yang and L.-Y. Xie, Org. Lett., 2025, 27, 8263–8268 CrossRef CAS.
- J. Corpas, P. Mauleón, R. G. Arrayás and J. C. Carretero, ACS Catal., 2021, 11, 7513–7551 CrossRef CAS.
- J. Chen, X. Bai, H. Jiang, C. Zhao, Y. Li, M. Chu, Y. Li, M. Zhang and L. Chen, Chem. Commun., 2024, 60, 10196–10199 RSC.
- L. Liu, K. Sun, L. Su, J. Dong, L. Cheng, X. Zhu, C.-T. Au, Y. Zhou and S.-F. Yin, Org. Lett., 2018, 20, 4023–4027 CrossRef CAS PubMed.
- L. Song, C. Ma, J. Huang, Y. Lv, H. Yue, J. You, W. Wei and D. Yi, J. Org. Chem., 2024, 89, 10974–10986 CrossRef CAS.
- W. Ding, J. Chai, C. Wang, J. Wu and N. Yoshikai, J. Am. Chem. Soc., 2020, 142, 8619–8624 CrossRef CAS PubMed.
- S. Dutta and A. K. Sahoo, Angew. Chem., Int. Ed., 2023, 62, e202300610 CrossRef CAS.
- H. Meng, S. Bai, Y. Qiao, T. He, W. Li and J. Ming, Org. Lett., 2022, 24, 5480–5485 Search PubMed.
- H. Zuo, E. Irran, H. F. T. Klare and M. Oestreich, Angew. Chem., Int. Ed., 2024, 63, e202401599 Search PubMed.
- Z. Li, A. García-Domínguez and C. Nevado, Angew. Chem., Int. Ed., 2016, 55, 6938–6941 CrossRef CAS.
- A. García-Domínguez, S. Müller and C. Nevado, Angew. Chem., Int. Ed., 2017, 56, 9949–9952 CrossRef PubMed.
- L. Guo, F. Song, S. Zhu, H. Li and L. Chu, Nat. Commun., 2018, 9, 4543 CrossRef PubMed.
- Z. Yang and R. M. Koenigs, Chem.–Eur. J., 2021, 27, 3694–3699 CrossRef CAS.
- S. Maiti and J. H. Rhlee, Chem. Commun., 2021, 57, 11346–11349 RSC.
- Y.-Z. Zhan, H. Meng and W. Shu, Chem. Sci., 2022, 13, 4930–4935 RSC.
- Q. Wang, X. Wang, Y. Liu, J. Zhang, J. Song and C. Guo, J. Am. Chem. Soc., 2025, 147, 8917–8927 CrossRef CAS.
- M.-D. Wang, Y.-N. Li, L.-X. Xiao, R.-H. Zhang, M. Yuan, Z.-L. Wang, X.-G. Zhang and H.-Y. Tu, Org. Lett., 2025, 27, 391–395 CrossRef CAS.
- S. Chang, C. Guo, R.-M. Zhong, Y.-L. Lai, H. Guo and L. Huang, Org. Lett., 2024, 26, 3733–3738 CrossRef CAS.
- Y. Li, Q. Shao, H. He, C. Zhu, X.-S. Xue and J. Xie, Nat. Commun., 2022, 13, 10 CrossRef CAS PubMed.
- W. Li, S. Yu, J. Li and Y. Zhao, Angew. Chem., Int. Ed., 2020, 59, 14404–14408 CrossRef CAS PubMed.
- R. Alfaro, A. Parra, J. Alemán, J. L. García Ruano and M. Tortosa, J. Am. Chem. Soc., 2012, 134, 15165–15168 Search PubMed.
- H. F. T. Klare, L. Albers, L. Süsse, S. Keess, T. Müller and M. Oestreich, Chem. Rev., 2021, 121, 5889–5985 CrossRef CAS PubMed.
- I. Nakamura, T. Sato and Y. Yamamoto, Angew. Chem., Int. Ed., 2006, 45, 4473–4475 Search PubMed.
- M. Iwasaki, D. Fujino, T. Wada, A. Kondoh, H. Yorimitsu and K. Oshima, Chem.–Asian J., 2011, 6, 3190–3194 CrossRef CAS.
- J. F. Hooper, A. B. Chaplin, C. González-Rodríguez, A. L. Thompson, A. S. Weller and M. C. Willis, J. Am. Chem. Soc., 2012, 134, 2906–2909 Search PubMed.
- T. Inami, T. Kurahashi and S. Matsubara, Chem. Commun., 2014, 51, 1285–1288 RSC.
- M. Iwasaki, N. Topolovčan, H. Hu, Y. Nishimura, G. Gagnot, R. Na nakorn, R. Yuvacharaskul, K. Nakajima and Y. Nishihara, Org. Lett., 2016, 18, 1642–1645 CrossRef CAS PubMed.
- H. Kim, J. Jang and S. Shin, J. Am. Chem. Soc., 2020, 142, 20788–20795 CrossRef CAS.
- M. Arambasic, J. F. Hooper and M. C. Willis, Org. Lett., 2013, 15, 5162–5165 CrossRef CAS.
- T. Delcaillau, H. L. Schmitt, P. Boehm, E. Falk and B. Morandi, ACS Catal., 2022, 12, 6081–6091 CrossRef CAS.
- H. F. T. Klare and M. Oestreich, J. Am. Chem. Soc., 2021, 143, 15490–15507 CrossRef CAS.
- Q. Wu, Z.-W. Qu, L. Omann, E. Irran, H. F. T. Klare and M. Oestreich, Angew. Chem., Int. Ed., 2018, 57, 9176–9179 CrossRef CAS PubMed.
- Q. Wu, E. Irran, R. Müller, M. Kaupp, H. F. T. Klare and M. Oestreich, Science, 2019, 365, 168–172 Search PubMed.
- T. He, H. F. T. Klare and M. Oestreich, Nature, 2023, 623, 538–543 Search PubMed.
- T. Randt, M. Lehmann, E. Irran, M. Kaupp, H. F. T. Klare and M. Oestreich, Nat. Chem., 2025, 17, 1666–1672 CrossRef CAS PubMed.
- M. Hanack, Acc. Chem. Res., 1976, 9, 364–371 CrossRef CAS.
- V. D. Nefedov, E. N. Sinotova and V. P. Lebedev, Russ. Chem. Rev., 1992, 61, 283–296 CrossRef.
- M. Niggemann and S. Gao, Angew. Chem., Int. Ed., 2018, 57, 16942–16944 CrossRef CAS.
- S. Popov, B. Shao, A. L. Bagdasarian, B. Wigman and H. M. Nelson, Synlett, 2020, 31, 1851–1856 Search PubMed.
- X.-J. Liu, Y. Xu, C. Tang, P.-C. Qian and L.-W. Ye, Sci. China Chem., 2022, 65, 20–30 CrossRef CAS.
- M.-L. Y. Riu, S. Popov, B. Wigman, Z. Zhao, J. Wong, K. N. Houk and H. M. Nelson, Eur. J. Org Chem., 2024, 27, e202400567 CrossRef CAS.
- S. Popov, B. Shao, A. L. Bagdasarian, T. R. Benton, L. Zou, Z. Yang, K. N. Houk and H. M. Nelson, Science, 2018, 361, 381–387 CrossRef CAS.
- T. He, H. F. T. Klare and M. Oestreich, J. Am. Chem. Soc., 2022, 144, 4734–4738 CrossRef CAS PubMed.
- B. Yu, A.-H. Liu, L.-N. He, B. Li, Z.-F. Diao and Y.-N. Li, Green Chem., 2012, 14, 957–962 Search PubMed.
- M. Nambo, Y. Maekawa and C. M. Crudden, ACS Catal., 2022, 12, 3013–3032 Search PubMed.
- L. Wang, W. He and Z. Yu, Chem. Soc. Rev., 2012, 42, 599–621 RSC.
- J. Lou, Q. Wang, P. Wu, H. Wang, Y.-G. Zhou and Z. Yu, Chem. Soc. Rev., 2020, 49, 4307–4359 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |