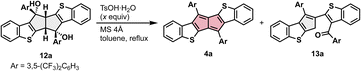Open Access Article
Open Access ArticleModular synthesis of benzothiophene-fused pentalenes reveals substituent-dependent antiaromaticity
Ryosuke
Isogai
 a,
Kosuke
Yasui†
a,
Kosuke
Yasui†
 b and
Aiko
Fukazawa
b and
Aiko
Fukazawa
 *b
*b
aDepartment of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Yoshida, Sakyo-ku, Kyoto 606-8501, Japan
bInstitute for Integrated Cell-Material Science (WPI-iCeMS), Institute for Advanced Study, Kyoto University, Yoshida, Sakyo-ku, Kyoto 606-8501, Japan. E-mail: afukazawa@icems.kyoto-u.ac.jp
First published on 12th January 2026
Abstract
Antiaromatic π-electron systems provide unique electronic features arising from the cyclic conjugation of 4n π-electrons, yet synthetic access to strongly antiaromatic heteroarene-fused scaffolds remains limited. Here we report a general synthetic route to benzothiophene-fused pentalenes via the first thiophene analogue of Brand's bicyclo[3.3.0]octadiene-2,6-dione intermediate. The pre-installation of the bicyclic five-membered-ring core at an early stage of synthesis enables efficient annulation of benzothiophene moieties and late-stage diversification at the 1,4-positions through the 1,2-addition of organometallic nucleophiles, followed by optimized dehydration. This strategy affords a series of benzothiophene-fused pentalenes bearing diverse aryl, heteroaryl, and alkynyl substituents in practical yields, with isolation by simple filtration. The benzothiophene-fused pentalenes thus obtained exhibit strong antiaromatic character that correlates with electronic effects, consistent with the topological charge stabilization rule. This work establishes a versatile platform for probing substituent-dependent antiaromaticity and for designing functional materials based on strongly antiaromatic π-systems.
Introduction
Antiaromatic π-electron systems exhibit distinct electronic properties such as narrow HOMO–LUMO gaps,1 amphoteric redox behavior,2 and low-lying triplet excited states,3,4 making them attractive frameworks for optoelectronic materials.5 Among these, pentalene (1), a bicyclic conjugated hydrocarbon with two fused five-membered rings, is a prototypical antiaromatic framework (Fig. 1a, top).6 Owing to its planarity and minimal bond-angle strain among 4n π-electron hydrocarbons, pentalene and its derivatives have been considered promising building blocks for functional materials.7 To exploit the characteristic properties of pentalene derived from its antiaromatic character, a variety of annulated derivatives have been developed.8–33 Dibenzo[a,e]pentalene (2) and its derivatives have been most extensively investigated due to their high stability,8–23 although the strong aromatic character of benzene rings diminishes the antiaromatic character of the pentalene cores (Fig. 1a, bottom left). In contrast, the annulation with weakly aromatic heteroarenes such as thiophene preserves the antiaromatic character of pentalene,24,25 giving rise to dithieno[a,e]pentalene (3),11a,12b,25,26 benzothiophene-fused (4), and its π-expanded analogues22e as thermally stable compounds (Fig. 1a, bottom right). These heteroarene-fused pentalenes offer an attractive platform for optoelectronic materials, yet their synthesis remains challenging due to the lack of general and efficient synthetic methods. | ||
| Fig. 1 (a) Effect of fused aromatic rings on the antiaromaticity of pentalene 1. (b) Previous synthetic methodologies of dibenzo[a,e]pentalenes 2. (c) Synthetic strategy in the present study. | ||
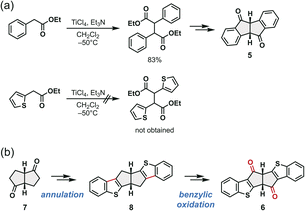
Previous synthetic routes for dibenzo[a,e]pentalenes were mostly based on intra- or intermolecular cyclization of phenylacetylenes (Fig. 1b).9–18 However, analogous reactions with thiophene-based substrates have generally failed or afforded poor yields (Fig. 1b).25,31 We hypothesized that these difficulties stem primarily from increased ring strain and higher reactivity of strongly antiaromatic dithieno[a,e]pentalene under the reaction conditions. Indeed, our recent studies indicated that benzothiophene-fused pentalenes can strongly coordinate to Ni(0),34 inhibiting the desired reaction.
To overcome these limitations, we revisited the classical strategy, which constructs the pentalene framework from a benzannulated bicyclo[3.3.0]octadiene-2,6-dione precursor (5, Fig. 1b). This method, originally reported by Brand in 1912,8 and recently refined by Esser and co-workers,20,21 enables transition metal-free formation of the dibenzo[a,e]pentalene framework. We herein report the development of a new synthetic route of benzothiophene-fused pentalenes 4via the corresponding benzothiophene-fused bicyclo[3.3.0]octadiene-2,6-dione intermediate (6) derived from bicyclo[3.3.0]octane-2,6-dione 7 (Fig. 1c). This approach allows late-stage introduction of diverse substituents to the 1,4-positions of the pentalene core, affording a new family of stable yet strongly antiaromatic compounds. The resulting compounds provide a versatile platform for elucidating the substituent effects on antiaromaticity and the corresponding physical properties.
Results and discussion
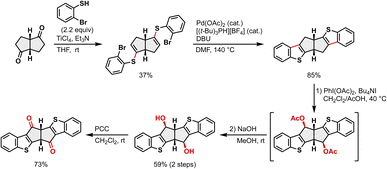
In Esser's improved synthesis of dibenzo[a,e]pentalene derivatives based on Brand's approach, the key intermediate, a benzannulated bicyclo[3.3.0]octadiene-2,6-dione 5, was prepared in only three steps via dimerization of ethyl phenylacetate followed by hydrolysis and intramolecular cyclization (Fig. 2a, top).20a,22h However, our attempts to apply these conditions to ethyl thienylacetate failed, yielding a complex mixture rather than the desired dimer (Fig. 2a, bottom), indicating the need for an alternative synthetic strategy to access thiophene-fused analogues.We therefore focused on the synthesis of benzothiophene-fused pentalene 4 through a new benzothiophene analogue of Brand's intermediate (6; Fig. 2b). Bicyclo[3.3.0]octane-2,6-dione (7) was chosen as the key starting material, as it embeds the fused five-membered-ring motif of the pentalene core from the outset.35 Introducing the principal source of ring strain at an early stage of synthesis avoids the strain accumulation that hampers conventional approaches in which the pentalene core is constructed only at the end. The benzothiophene rings were to be constructed laterally around this scaffold to furnish the hexacyclic compound 8, followed by benzylic oxidation to the desired diketone 6.
Following this strategy, a benzothiophene analogue of Brand's intermediate, 6, could be successfully synthesized as shown in Scheme 1. The Ti-mediated nucleophilic addition of 2-bromobenzothiol to 7 and subsequent dehydration36 afforded the alkenyl sulfide 9 in 37% yield. A Pd-catalyzed intramolecular cyclization efficiently produced the benzothiophene-fused bicyclo[3.3.0]octadiene 8 in 85% yield.‡ Regioselective benzylic oxidation of 8 proved challenging, yet PhI(OAc)2/TBAI37 enabled regioselective conversion to the acetoxylated intermediate 10, which upon hydrolysis gave the desired diol 11 in 59% overall yield for two steps. Finally, PCC oxidation of 11 gave the desired diketone 6 in 73% yield. This compound represents the first thiophene-annulated analogue of Brand's intermediate and a versatile precursor to pentalene frameworks.
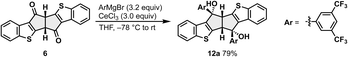
We next examined the construction of benzothiophene-fused pentalenes from 6. The 1,2-addition of Grignard reagents to 6 proceeded smoothly in the presence of CeCl3 under conditions essentially the same as those reported for the synthesis of dibenzo[a,e]pentalene derivatives.20a However, subsequent dehydration gave a different outcome, indicating that modification of the reaction conditions was required (Scheme 2). Specifically, for the preparation of benzothiophene-fused pentalene 4a bearing 3,5-bis(trifluoromethyl)phenyl groups as a model compound, the corresponding Grignard reagent was subjected to the reaction with 6 in the presence of CeCl3 to afford the bis(benzyl alcohol) 12a in 79% yield (Scheme 2). In contrast, the following dehydration using the catalytic amount of TsOH·H2O produced the desired pentalene 4a in only 17% yield, with a ring-opened compound 13a as the major product in 45% yield (entry 1 in Table 1).§ A similar ring-opening reaction has been reported in the synthesis of a highly strained cyclophane-type dibenzo[a,e]pentalene.21a In line with this observation, the low yield of 4a accompanied by the ring opening suggests that thiophene fusion introduces additional strain. Increasing the amount of TsOH·H2O reversed the product ratio (entries 2 and 3), and 3.0 equivalents afforded the desired pentalene 4a in 74% yield (entry 3).
With the optimized conditions, diverse benzothiophene-fused pentalenes 4 were synthesized from the common intermediate 6 by 1,2-addition of the corresponding Grignard reagents or organolithium reagents in the presence of CeCl3 (Fig. 3). The scope includes derivatives bearing electron-withdrawing 3,5-(CF3)2C6H3 (4a), relatively neutral (Ph, 4b), and electron-donating (4-MeOC6H4, 4c) aryl groups, as well as heteroaryl (2-thienyl, 4d), and triisopropylsilyl (TIPS)ethynyl groups (4e). Notably, the final dehydration step induced precipitation of desired products 4, enabling facile isolation by simple filtration without chromatography. This route thus provides practical access to structurally diverse benzothiophene-fused pentalenes.
Single-crystal X-ray diffraction of 4e unambiguously confirmed the formation of benzothiophene-fused pentalenes (Fig. 3c and S1–S2). The crystal structure of 4b has been reported previously.34 Both 4b and 4e featured a highly planar benzothiophene-fused pentalene core, forming slipped π–π stacking arrangements along the molecular long axis (Fig. S2). The peripheral C–C bonds of the pentalene cores displayed pronounced bond-length alternation, consistent with strong antiaromatic character. The C–C bonds fused to the thiophene rings were relatively short, indicating double-bond character.
The UV/vis/NIR absorption spectra of 4a–e exhibited weak, broad first absorption bands at around 600–1100 nm, typical of cyclic 4n π-electron systems, along with a more intense second band around 500 nm (Fig. 4a). Compared with dibenzo[a,e]pentalene (2, R = SiMe3, λmax = 503 nm)25 and dithieno[a,e]pentalene (3, R = SiMe3, λmax = 639 nm),25 the first absorption bands of 4a–e (λmax at around 800 nm) were markedly red-shifted, reflecting enhanced antiaromatic character of the pentalene core and π-expansion. Cyclic voltammetry showed reversible oxidation and reduction waves (Fig. 4b), confirming their amphoteric redox behavior.
The redox potentials reflected the electronic nature of the substituents: the most electron-withdrawing 4a exhibited E1/2,ox = +0.48 V and E1/2,red = −0.97 V (vs. Fc/Fc+), whereas the electron-donating 4c showed the half-wave potentials E1/2,ox = +0.16 V and E1/2,red = −1.36 V (vs. Fc/Fc+), respectively. These results demonstrate that the electronic properties of benzothiophene-fused pentalene can be finely tuned by varying the substituents. The HOMO–LUMO gaps estimated from redox onsets (1.17–1.37 eV, Table S2) were significantly smaller than those of 2 (2.48 eV)25 and 3 (1.87 eV),25 consistent with the enhanced antiaromatic character and efficient π-conjugation.
To quantify the degree of antiaromatic character, the paratropicity strength, a magnetic descriptor of antiaromaticity, was estimated by computing the NICS(1.7)zz values38 for 4a–d and 4e′ at the M06-2X/6-31++G(2d,p)//M06-2X/6-31++G(d) level of theory (Fig. 5a).¶ All compounds exhibited large positive values in the range from +19.2 ppm to +24.4 ppm, confirming pronounced antiaromaticity relative to dibenzo[a,e]pentalene 2 (R = Ph, +3.8 ppm). The anisotropy of the induced current density (ACID) plot39 for 4a calculated at the same level of theory clearly indicated that a paramagnetic ring current exists along the 8-membered-ring periphery of the pentalene moiety (Fig. 5b). Notably, the trend in NICS(1.7)zz values, 4c (+19.2 ppm) < 4d (+19.8 ppm) < 4b (+20.3 ppm) < 4a (+23.6 ppm) < 4e′ (+24.4 ppm), intuitively correlates with the electron-withdrawing strength of the substituents. This result is consistent with recent observations in alkylthio-substituted dibenzo[a,e]pentalene and its sulfone analogue,20e and underscores the importance of the electronic effects of substituents in modulating antiaromaticity.
To rationalize these substituent effects, we applied the topological charge stabilization rule, originally proposed by Gimarc40 and recently utilized by Wu et al., to understand the positional effects of heteroatom doping on the paratropicity of indacene-based PAHs.41 According to this rule, the 1,4-carbons of pristine pentalene possess partial positive character (δ+); thus, electron-donating substituents stabilize the system, while electron-withdrawing groups destabilize it (Fig. 5c). Consequently, the former are expected to weaken, and the latter to enhance, the antiaromatic character. This interpretation is consistent with the observed trend in NICS values and provides an intuitive explanation for the substituent-dependent modulation of the antiaromatic character of pentalene. These results demonstrate that the electronic effects of both the fused ring system and the substituents cooperatively determine the degree of antiaromatic character of the pentalene moiety. In particular, the introduction of strongly electron-withdrawing groups effectively enhances the antiaromatic character of the pentalene core.
Conclusions
In conclusion, we developed a versatile and general synthetic route to benzothiophene-fused pentalenes through a thiophene analogue of Brand's bicyclo[3.3.0]octadiene-2,6-dione intermediate. Pre-installation of the bicyclic five-membered-ring framework enabled efficient annulation of benzothiophene units and late-stage diversification at the 1,4-positions, overcoming the limitations of conventional approaches to hereroarene-fused pentalenes. The resulting compounds constitute a new family of stable yet strongly antiaromatic compounds, whose electronic and magnetic properties can be finely tuned by substituents. These findings provide a robust platform for understanding substituent effects on antiaromaticity and offer design principles for future functional materials based on antiaromatic π-systems.This work was supported by JST CREST Grant Number JPMJCR23O2 (for A.F.), and JSPS KAKENHI Grant Numbers JP20H05864, JP21H01916, and JP24H00458 (for A.F.), and JP24KJ1436 (for R.I.). The authors thank the iCeMS Analysis Center for providing access to the NMR spectrometer. The synchrotron single-crystal X-ray diffraction measurements were performed at the BL02B1 beamline of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI; project no. 2024A1699).
Author contributions
R. I.: investigation, methodology, formal analysis, data curation, funding acquisition, visualization, writing – original draft. K. Y.: conceptualization, supervision, writing – review & editing. A. F.: conceptualization, data curation, formal analysis, funding acquisition, project administration, supervision, visualization, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
Data supporting this article are included in the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5sc09325b.CCDC 2504966 (4e) contains the supplementary crystallographic data for this paper.42
Notes and references
- A. Minsky, A. Y. Meyer and M. Rabinovitz, Tetrahedron, 1985, 41, 785–791 CrossRef CAS.
- I. Willner, J. Y. Becker and M. Rabinovitz, J. Am. Chem. Soc., 1979, 101, 395–401 CrossRef CAS.
- (a) W. T. Borden, E. R. Davidson and P. Hart, J. Am. Chem. Soc., 1978, 100, 388–392 CrossRef CAS; (b) J. A. Jafri and M. D. Newton, J. Am. Chem. Soc., 1978, 100, 5012–5017 CrossRef CAS; (c) M. Eckert-Maksić, M. Vazdar, M. Barbatti, H. Lischka and Z. B. Maksić, J. Chem. Phys., 2006, 125, 064310 Search PubMed; (d) G. Hohlneicher, L. Packschies and J. Weber, Phys. Chem. Chem. Phys., 2007, 9, 2517–2530 RSC; (e) P. B. Karadakov, J. Phys. Chem. A, 2008, 112, 7303–7309 CrossRef CAS PubMed; (f) J. I.-C. Wu, Y. Mo, F. A. Evangelista and P. v. R. Schleyer, Chem. Commun., 2012, 48, 8437–8439 RSC.
- (a) J. Wirz, A. Krebs, H. Schmalstieg and H. Angliker, Angew Chem. Int. Ed. Engl., 1981, 20, 192–193 CrossRef; (b) M. Ueda, K. Jorner, Y. M. Sung, T. Mori, Q. Xiao, D. Kim, H. Ottosson, T. Aida and Y. Itoh, Nat. Commun., 2017, 8, 346 CrossRef PubMed; (c) A. Kostenko, B. Tumanskii, Y. Kobayashi, M. Nakamoto, A. Sekiguchi and Y. Apeloig, Angew. Chem., Int. Ed., 2017, 56, 10183–10187 CrossRef CAS PubMed.
- Selected reviews: (a) A. Konishi and M. Yasuda, Chem. Lett., 2021, 50, 195 CrossRef CAS; (b) C. Hong, J. Baltazar and J. D. Tovar, Eur. J. Org Chem., 2022, e202101343 CrossRef CAS.
- (a) K. Hafner and H. U. Süss, Angew Chem. Int. Ed. Engl., 1973, 12, 575–577 Search PubMed; (b) K. Hafner, R. Dönges, E. Goedecke and R. Kaiser, Angew Chem. Int. Ed. Engl., 1973, 12, 337–339 CrossRef; (c) R. Dönges, K. Hafner and H. J. Lindner, Tetrahedron Lett., 1976, 17, 1345–1348 Search PubMed; (d) M. Suda and K. Hafner, Tetrahedron Lett., 1977, 18, 2449–2452 Search PubMed; (e) T. Bally, S. Chai, M. Neuenschwander and Z. Zhu, J. Am. Chem. Soc., 1997, 119, 1869–1875 Search PubMed.
- H. Hopf, Angew. Chem., Int. Ed., 2013, 52, 12224–12226 Search PubMed.
- K. Brand, Berichte Dtsch. Chem. Ges., 1912, 45, 3071–3077 Search PubMed.
- (a) F. Xu, L. Peng, A. Orita and J. Otera, Org. Lett., 2012, 14, 3970–3973 CrossRef CAS PubMed; (b) F. Xu, L. Peng, K. Shinohara, T. Morita, S. Yoshida, T. Hosoya, A. Orita and J. Otera, J. Org. Chem., 2014, 79, 11592–11608 CrossRef CAS PubMed.
- M. Saito, M. Nakamura, T. Tajima and M. Yoshioka, Angew. Chem., Int. Ed., 2007, 46, 1504–1507 CrossRef CAS PubMed.
- (a) Z. U. Levi and T. D. Tilley, J. Am. Chem. Soc., 2009, 131, 2796–2797 Search PubMed; (b) Z. U. Levi and T. D. Tilley, J. Am. Chem. Soc., 2010, 132, 11012–11014 Search PubMed; (c) J. Shen, D. Yuan, Y. Qiao, X. Shen, Z. Zhang, Y. Zhong, Y. Yi and X. Zhu, Org. Lett., 2014, 16, 4924–4927 CrossRef CAS PubMed.
- (a) T. Kawase, A. Konishi, Y. Hirao, K. Matsumoto, H. Kurata and T. Kubo, Chem.–Eur. J., 2009, 15, 2653–2661 CrossRef CAS PubMed; (b) A. Konishi, T. Fujiwara, N. Ogawa, Y. Hirao, K. Matsumoto, H. Kurata, T. Kubo, C. Kitamura and T. Kawase, Chem. Lett., 2010, 39, 300–301 Search PubMed.
- H. Zhang, T. Karasawa, H. Yamada, A. Wakamiya and S. Yamaguchi, Org. Lett., 2009, 11, 3076–3079 CrossRef CAS PubMed.
- (a) A. S. K. Hashmi, M. Wieteck, I. Braun, P. Nösel, L. Jongbloed, M. Rudolph and F. Rominger, Adv. Synth. Catal., 2012, 354, 555–562 Search PubMed; (b) M. M. Hansmann, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2013, 52, 2593–2598 CrossRef CAS PubMed; (c) T. Wurm, E. C. Rüdiger, J. Schulmeister, S. Koser, M. Rudolph, F. Rominger, U. H. F. Bunz and A. S. K. Hashmi, Chem.–Eur. J., 2018, 24, 2735–2740 Search PubMed; (d) S. Tavakkolifard, K. Sekine, L. Reichert, M. Ebrahimi, K. Museridz, E. Michel, F. Rominger, R. Babaahmadi, A. Ariafard, B. F. Yates, M. Rudolph and A. S. K. Hashmi, Chem.–Eur. J., 2019, 25, 12180–12186 CrossRef CAS.
- P. R. Fuentes, M. v. W. Rekowski, W. B. Schweizer, J. P. Gisselbrecht, C. Boudon and F. Diedrich, Org. Lett., 2012, 14, 4066–4069 CrossRef PubMed.
- C. Chen, M. Harhausen, R. Liedtke, K. Bussmann, A. Fukazawa, S. Yamaguchi, J. L. Petersen, C. G. Daniliuc, R. Fröhlich, G. Kehr and G. Erker, Angew. Chem., Int. Ed., 2013, 52, 5992–5996 CrossRef CAS PubMed.
- T. Maekawa, Y. Segawa and K. Itami, Chem. Sci., 2013, 4, 2369–2373 RSC.
- K. Takahashi, S. Ito, R. Shintani and K. Nozaki, Chem. Sci., 2017, 8, 101–107 Search PubMed.
- Y. Wu, Y. Wang, J. Chen, G. Zhang, J. Yao, D. Zhang and H. Fu, Angew. Chem., Int. Ed., 2017, 56, 9400–9404 CrossRef CAS.
- (a) J. Wilbuer, D. C. Grenz, G. Schnakenburg and B. Esser, Org. Chem. Front., 2017, 4, 658–663 Search PubMed; (b) D. C. Grenz, M. Schmidt, D. Kratzert and B. Esser, J. Org. Chem., 2018, 83, 656–663 Search PubMed; (c) M. Hermann, T. Böttcher, M. Schorpp, S. Richert, D. Wassy, I. Krossing and B. Esser, Chem.–Eur. J., 2021, 27, 4964–4970 CrossRef CAS; (d) M. Schmidt, L. A. Vicente, M. T. González, L. A. Zotti, B. Esser and E. Leary, Chem.–Eur. J., 2024, 30, e202400935 Search PubMed; (e) M. Hermann, S. M. A. Battisti and B. Esser, Asian J. Org. Chem., 2025, 14, e202500231 CrossRef CAS.
- (a) M. Hermann, D. Wassy, D. Kratzert and B. Esser, Chem.–Eur. J., 2018, 24, 7374–7387 Search PubMed; (b) D. Wassy, M. Pfeifer and B. Esser, J. Org. Chem., 2020, 85, 34–43 CrossRef CAS PubMed; (c) D. Wassy, M. Hermann, J. S. Wössner, L. Frédéric, G. Pieters and B. Esser, Chem. Sci., 2021, 12, 10150–10158 Search PubMed; (d) J. S. Wössner, D. Wassy, A. Weber, M. Bovenkerk, M. Hermann, M. Schmidt and B. Esser, J. Am. Chem. Soc., 2021, 143, 12244–12252 CrossRef; (e) J. S. Wössner, J. Kohn, D. Wassy, M. Hermann, S. Grimme and B. Esser, Org. Lett., 2022, 24, 983–988 Search PubMed; (f) M. Hermann, D. Wassy, J. Kohn, P. Seitz, M. U. Betschart, S. Grimme and B. Esser, Angew. Chem., Int. Ed., 2021, 60, 10680–10689 CrossRef CAS PubMed; (g) P. Seitz, L. Rzesny, D. Busse, X. Xiang, M. Hermann, L. Estaque, G. Pieters and B. Esser, J. Am. Chem. Soc., 2025, 147, 41610–41619 Search PubMed; (h) A. Gupta, P. Seitz, M. Hermann, B. Esser and R. Naaman, Adv. Funct. Mater., 2025 DOI:10.1002/adfm.202523339.
- (a) T. Kawase, T. Fujiwara, C. Kitamura, A. Konishi, Y. Hirao, K. Matsumoto, H. Kurata, T. Kubo, S. Shinamura, H. Mori, E. Miyazaki and K. Takimiya, Angew. Chem., Int. Ed., 2010, 49, 7728–7732 Search PubMed; (b) M. Nakano, I. Osaka, K. Takimiya and T. Koganezawa, J. Mater. Chem. C, 2013, 2, 64–70 RSC; (c) M. Nakano, I. Osaka and K. Takimiya, J. Mater. Chem. C, 2015, 3, 283–290 RSC; (d) G. Dai, J. Chang, W. Zhang, S. Bai, K.-W. Huang, J. Xu and C. Chi, Chem. Commun., 2014, 51, 503–506 Search PubMed; (e) G. Dai, J. Chang, X. Shi, W. Zhang, B. Zheng, K.-W. Huang and C. Chi, Chem.–Eur. J., 2014, 21, 2019–2028 CrossRef PubMed; (f) G. Dai, J. Chang, J. Luo, S. Dong, N. Aratani, B. Zheng, K. Huang, H. Yamada and C. Chi, Angew. Chem., Int. Ed., 2016, 55, 2693–2696 CrossRef CAS PubMed; (g) G. Dai, J. Chang, L. Jing and C. Chi, J. Mater. Chem. C, 2016, 4, 8758–8764 RSC; (h) M. Hermann, R. Wu, D. C. Grenz, D. Kratzert, H. Li and B. Esser, J. Mater. Chem. C, 2018, 6, 5420–5426 RSC.
- (a) J. Sprachmann, T. Wachsmuth, M. Bhosale, D. Burmeister, G. J. Smales, M. Schmidt, Z. Kochovski, N. Grabicki, R. Wessling, E. J. W. List-Kratochvil, B. Esser and O. Dumele, J. Am. Chem. Soc., 2023, 145, 2840–2851 CrossRef CAS PubMed; (b) P. Seitz, M. Bhosale, L. Rzesny, A. Uhlmann, J. S. Wössner, R. Wessling and B. Esser, Angew. Chem., Int. Ed., 2023, 62, e202306184 CrossRef CAS.
- C. K. Frederickson, L. N. Zakharov and M. M. Haley, J. Am. Chem. Soc., 2016, 138, 16827–16838 CrossRef CAS PubMed.
- J. Usuba, M. Hayakawa, S. Yamaguchi and A. Fukazawa, Chem.–Eur. J., 2021, 27, 1638–1647 CrossRef CAS.
- C. Hu and Q. Zhang, Chin. J. Chem., 2013, 31, 1404–1408 Search PubMed.
- S. Kato, S. Kuwako, N. Takahashi, T. Kijima and Y. Nakamura, J. Org. Chem., 2016, 81, 7700–7710 CrossRef CAS.
- H. Oshima, A. Fukazawa and S. Yamaguchi, Angew. Chem., Int. Ed., 2017, 56, 3270–3274 Search PubMed.
- (a) B. Yuan, J. Zhuang, K. M. Kirmess, C. N. Bridgmohan, A. C. Whalley, L. Wang and K. N. Plunkett, J. Org. Chem., 2016, 81, 8312–8318 Search PubMed; (b) A. Konishi, S. Satake and M. Yasuda, Chem. Lett., 2020, 49, 589–592 Search PubMed.
- X. Yin, Y. Li, Y. Zhu, Y. Kan, Y. Li and D. Zhu, Org. Lett., 2011, 13, 1520–1523 CrossRef CAS PubMed.
- T. Gazdag, P. J. Mayer, P. P. Kalapos, T. Holczbauer, O. E. Bakouri and G. London, ACS Omega, 2022, 7, 8336–8349 CrossRef CAS PubMed.
- (a) A. Konishi, Y. Okada, M. Nakano, K. Sugisaki, K. Sato, T. Takui and M. Yasuda, J. Am. Chem. Soc., 2017, 139, 15284–15287 CrossRef CAS PubMed; (b) A. Konishi, Y. Okada, R. Kishi, M. Nakano and M. Yasuda, J. Am. Chem. Soc., 2019, 141, 560–571 CrossRef CAS PubMed; (c) A. Konishi, K. Horii, H. Iwasa, Y. Okada, R. Kishi, M. Nakano and M. Yasuda, Chem.–Asian J., 2021, 16, 1553–1561 Search PubMed; (d) K. Horii, A. Nogata, Y. Mizuno, H. Iwasa, M. Suzuki, K. Nakayama, A. Konishi and M. Yasuda, Chem. Lett., 2022, 51, 325–329 CrossRef CAS; (e) Y. Mizuno, A. Nogata, M. Suzuki, K. Nakayama, I. Hisaki, R. Kishi, A. Konishi and M. Yasuda, J. Am. Chem. Soc., 2023, 145, 20595–20609 CrossRef CAS PubMed.
- Q. Jiang, T. Wang, Y. Han, H. Wei, Y. Deng, Y. Geng and C. Chi, Angew. Chem., Int. Ed., 2025, e202513158 CAS.
- R. Kuwata, T. Imanishi, S. Hasegawa, Z. Wang, J. Usuba, K. Yasui, M. U. G. Khan, J. I. Wu, Y. Uetake and A. Fukazawa, ChemRxiv, 2025 DOI:10.2634/chemrxiv-2025-l8c87-v2.
- R. M. Moriarty, M. P. Duncan, R. K. Vaid and O. M. Prakash, Org. Synth., 1990, 68, 175 CrossRef CAS.
- T. Mukaiyama and K. Saigo, Chem. Lett., 1973, 2, 479–482 CrossRef.
- H. Zaimoku, T. Hatta, T. Taniguchi and H. Ishibashi, Org. Lett., 2012, 14, 6088–6091 Search PubMed.
- Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta and P. v. R. Schleyer, Chem. Rev., 2005, 105, 3842–3888 CrossRef CAS PubMed.
- D. Geuenich, K. Hess, F. Köhler and R. Herges, Chem. Rev., 2005, 105, 3758–3772 CrossRef CAS PubMed.
- B. M. Gimarc, J. Am. Chem. Soc., 1983, 105, 1979–1984 CrossRef CAS.
- S. Jalife and J. I. Wu, J. Org. Chem., 2025, 90, 4012–4017 CrossRef CAS PubMed.
- CCDC 2504966: Experimental Crystal Structure Determination, 2026, DOI:10.5517/ccdc.csd.cc2q2mct.
- L. J. Karas, S. Jalife, R. V. Viesser, J. V. Soares, M. M. Haley and J. I. Wu, Angew. Chem., Int. Ed., 2023, 62, e202307379 CrossRef CAS PubMed.
Footnotes |
| † Present address: Department of Applied Chemistry, Graduate School of Engineering, The University of Osaka, Suita, Osaka 565-0871, Japan. |
| ‡ The yield of this intramolecular cyclization reaction was found to be sensitive to the type of ligand and base used in the reaction. The effect of the ligand and base is shown in Table S1. |
| § The proposed reaction mechanism for this ring-opening reaction is shown in Scheme S1. |
| ¶ X-ray crystal data were available only for compounds 4b and 4e. Geometry optimizations were therefore performed for 4a–d and 4e′, and the optimized structures were used for further analyses. To reduce computational costs, a model compound 4e′, in which the TIPS groups were replaced with TMS groups, was used instead of 4e. Several density functionals were tested using 4e′ as a model system, given that NICS values are sensitive to even subtle differences in bond-length distributions, as noted in ref. 43. As a result, the M06-2X density functional gave the best agreement with the experimental C–C bond lengths of the pentalene core determined by X-ray crystallographic analysis. See the SI for details. |
| This journal is © The Royal Society of Chemistry 2026 |