Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A donor–acceptor photosensitizer-catalyst dyad for light-driven nicotinamide hydrogenation
Alexander
Tombrink†
 a,
Mohini
Semwal†
bc,
Tamar
Maisuradze†
c,
Alexander K.
Mengele
a,
Mohini
Semwal†
bc,
Tamar
Maisuradze†
c,
Alexander K.
Mengele
 d,
Daniel
Straub
e,
Alexander J. C.
Kuehne
d,
Daniel
Straub
e,
Alexander J. C.
Kuehne
 e,
Sven
Rau
e,
Sven
Rau
 d,
Stephan
Kupfer
d,
Stephan
Kupfer
 c,
Benjamin
Dietzek-Ivanšić
c,
Benjamin
Dietzek-Ivanšić
 *bcf and
Birgit
Esser
*bcf and
Birgit
Esser
 *a
*a
aInstitute of Organic Chemistry II and Advanced Materials, Ulm University, Albert-Einstein-Allee 11, 89081 Ulm, Germany. E-mail: birgit.esser@uni-ulm.de
bLeibniz Institute of Photonic Technology, Research Department Functional Interfaces, Albert-Einstein-Str. 9, 07745 Jena, Germany. E-mail: benjamin.dietzek@uni-jena.de
cInstitute of Physical Chemistry, Friedrich Schiller University Jena, Helmholtzweg 4, 07743 Jena, Germany
dInstitute of Inorganic Chemistry I, Ulm University, Albert-Einstein-Allee 11, 89081 Ulm, Germany
eInstitute of Organic Chemistry III, Ulm University, Albert-Einstein-Allee 11, 89081 Ulm, Germany
fLeibniz-Institut für Oberflächenmodifizierung e.V. (IOM), Permoserstraße 15, 04318 Leipzig, Germany
First published on 23rd December 2025
Abstract
Using light energy to drive chemical transformations is of great relevance, with photosynthesis in nature as a grand example. In artificial light-driven catalysis, part of nature's complex supramolecular architecture can be mimicked through the so-called covalently linked photosensitizer-catalyst (PS-CAT) dyads. We herein report a dyad using an organic donor–acceptor PS, with dipyridophenazine as the acceptor and tert-butylcarbazole as the donor (2tBuCzDPPZ), that contains a coordination site for a rhodium(III)Cp* center as the catalyst. The organic PS shows a charge-transfer transition upon visible-light irradiation and has redox properties similar to typically used ruthenium-based PSs. The resulting PS-CAT dyad 2tBuCzDPPZRhCp* shows – with methoxy-substituted 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole (BIH-OMe) as the sacrificial electron donor – photocatalytic activity in light-driven NAD+ reduction with a TON of 3.2 (after 4 h). Femtosecond transient absorption and resonance Raman spectroscopy, as well as time-dependent density functional theory (TDDFT) calculations, shed light on the photophysical properties of the PS and PS-CAT dyad and reveal a high dependency of the photoluminescence quantum yield and excited state properties on solvent polarity – in line with its donor–acceptor structure. This work presents a new design concept for PS-CAT dyads in artificial light-driven catalysis and provides important insight into the interplay between solvation dynamics of organic donor–acceptor systems and their photophysics, paving the way for future design strategies.
Introduction
Nature's photosynthesis is arguably one of the most significant chemical transformation processes on earth, and it is consequently appealing for scientists to artificially recreate specific core processes in the laboratory.1 The interplay of light-harvesting and cascade-like energy transfer, photoinduced charge separation and catalytic turnover processes are of particular interest in this context, and researchers strive to exploit them for artificial systems. In homogeneous artificial light-driven catalysis, typically multicomponent systems are used, containing a photosensitizer (PS) and catalyst (CAT), among others, where the photoinduced electron transfer (PET) between these components is a key step in the photocatalytic cycle. Here, being of intermolecular nature, the PET is limited by the diffusional colliding of PS and CAT or sacrificial agents during the excited-state lifetime of the PS.2,3 By contrast, in nature a network of precisely positioned molecular cofactors within photosynthetic proteins efficiently couples light-harvesting, electron transfer and chemical transformation within a complex supramolecular architecture.4 Part of this complex assembly can be mimicked through the so-called PS-CAT dyads, in which the photosensitizer and the catalytically active center are covalently linked via a bridging unit.5–7 An example is the ruthenium-based PS [Ru(tbbpy)2(tpphz)]2+ (Rutpphz; tbbpy = 4,4′-di-tert-butyl-2,2′-bipyridine, tpphz = tetrapyrido[3,2-a:2′,3′-c:3″,2″-h:2‴,3‴j]phenazine), which provides a second α-diimine coordination site to attach various types of metal centers to form the respective PS-CAT architectures (Fig. 1a).8 By coordinating PdCl2, PtCl2, or PtI2, these dyads showed activity in the hydrogen-evolution reaction (HER) with TONs (turn-over numbers) of up to 276 after 70 h.5,9 By altering the catalytic center to RhCp*Cl (Cp*: 1,2,3,4,5-pentamethylcyclopentadienyl), the resulting dyad RutpphzRhCp* not only showed photocatalytic activity in the HER, but also in the reduction of the nicotinamide co-factor NAD+ with TONs up to 6 after 90 min.7,10 | ||
| Fig. 1 (a) Previously reported Ru-based dyad RutpphzRhCp* and (b) design of the donor–acceptor (D–A)-based photosensitizer (PS)-catalyst (CAT) dyad 2tBuCzDPPZRhCp*. | ||
A disadvantage of these dyads is the use of a Ru-based complex as a PS, containing a rare and expensive noble transition metal. Limited options for chemical modifications and low stability can also be problematic. Using organic chromophores as a PS that consist of abundant elements is an attractive goal, and has been realized on few occasions in PS-CAT dyads.6,11–14 Since the main first step in the light-driven catalysis of the Ru-based dyads is a 1MLCT population upon photon absorption, we searched for a suitable replacement in the form of an organic donor–acceptor (D–A) compound that also shows a vectorial charge-transfer transition upon visible-light irradiation and contains a coordination site for the CAT moiety. We choose a Rh(III)Cp* center as a model CAT as it offers a broad variety of known catalytic functions15–18 and has been investigated with a multitude of spectroscopic tools.10,19–21 The above mentioned coordination site can easily be employed for alternative CAT centers like PtI2 or PdCl2. We identified 2tBuCzDPPZ (bis(di-tert-butylcarbazole)dipyridophenazine), reported by Morgan et al., to match our requirements (blue/red structure in Fig. 1b). 2tBuCzDPPZ is an organic red-light-emitting compound with a high molar absorptivity, featuring a dipyrido unit to attach RhCp* as the catalytic center.22 In addition, its reported redox properties fit to earlier reported Ru-polypyridyl centers, as the oxidation potential of 0.94 V lies close to the oxidation of RuII to RuIII at around 0.8 V (all redox potentials given versus the ferrocene/ferrocenium redox couple (Fc/Fc+)). Also, the reported reduction potential of −1.59 V is close to the reduction of the bridging ligand at −1.44 V of the Ru-dyad.10,22 Furthermore, the dipyridophenazine coordination sphere provides an attractive interaction between the CAT-based metal center and the extended π-ligand system.23,24 This makes 2tBuCzDPPZ highly desirable as a purely organic PS for the novel PS-CAT dyad 2tBuCzDPPZRhCp* (Fig. 1b).10
We herein set out to synthesize and investigate the photocatalytic activity of the new PS-CAT dyad 2tBuCzDPPZRhCp*. We report its excited-state photophysics, in comparison to 2tBuCzDPPZ, using resonance Raman (rR) spectroscopy, femtosecond transient absorption spectroscopy, and quantum chemical calculations (DFT and TDDFT as well as TDA-TDDFT) including scalar-relativistic effects as well as spin–orbit couplings, revealing strong solvent-dependent variations in the triplet-state character and lifetimes. We demonstrate that 2tBuCzDPPZRhCp* – with a suitable sacrificial electron donor – shows photocatalytic activity in the light-driven NAD+ reduction. This work presents a new design concept for PS-CAT dyads in artificial light-driven catalysis since the TADF-like chromophore 2tBuCzDPPZ features the crucial property of directional light-induced electron/energy transfer within the PS towards the CAT entity. The understanding of the deactivation mechanism and proof of photocatalytic competence opens a pathway to improved properties in artificial photosynthesis.
Results and discussion
Synthesis
2tBuCzDPPZ was synthesized in three steps in analogy to the literature22 starting with a condensation reaction25 between 1,10-phenanthroline-5,6-dione and 2-amino-4,5-difluoroaniline to form 2FDPPZ (Scheme 1) complementing the π-system of the chromophore. In a subsequent nucleophilic aromatic substitution22 the two 3,6-di-tert-butyl-9H-carbazoles (tBuCz) were attached, furnishing 2tBuCzDPPZ. The PS-CAT dyad 2tBuCzDPPZRhCp* was obtained by reaction of the ligand with the pentamethylcyclopentadienyl rhodium(III)-dichloride dimer.11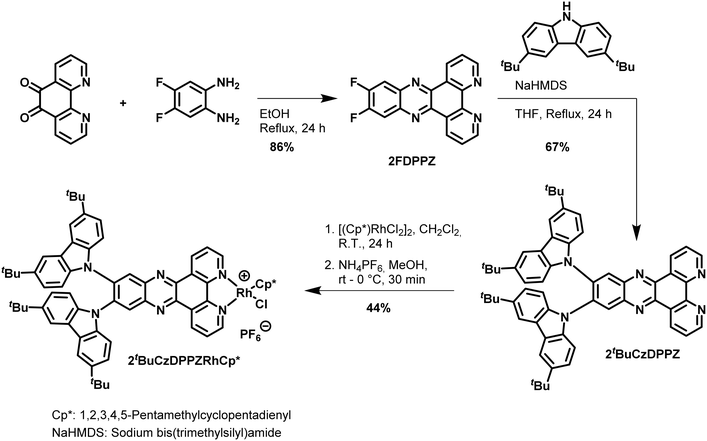 | ||
| Scheme 1 Synthesis of the D–A-photosensitizer/ligand 2tBuCzDPPZ and its Rh complex 2tBuCzDPPZRhCp* as a PS-CAT dyad. | ||
We also synthesized further modifications of the D–A-based PS-CAT dyad by varying the donor type and position (Scheme S1). The synthesis of the para-isomer p2tBuCzDPPZRhCp* started with a Buchwald–Hartwig amination of 4,7-dibromobenzo[c][1,2,5]thiadiazole with tBuCz (see the SI for details). A reduction of the thiadiazole with LiAlH4 gave the respective diamine, which was directly condensed with 1,10-phenanthroline-5,6-dione to the final ligand p2tBuCzDPPZ. This was then reacted with the pentamethylcyclopentadienyl rhodium dichloride dimer to form p2tBuCzDPPZRhCp*.11 For the third dyad 2TPADPPZRhCp*, 1,10-phenanthroline-5,6-dione was condensed with 4,5-dibromo-o-phenylenediamine to form 11,12-dibromodipyridophenazine, which was converted to 2TPADPPZ by Suzuki–Miyaura coupling with 4-diphenylaminophenylboronic acid (Scheme S1). Reaction with the pentamethylcyclopentadienyl rhodium dichloride dimer afforded 2TPADPPZRhCp*.11
All compounds were characterized by NMR spectroscopy and high-resolution mass spectrometry (see SI Fig. S1–S24).
In the following section, we report the detailed optoelectronic and photophysical characterization and catalyst optimization of the PS-CAT dyad 2tBuCzDPPZRhCp* as well as the ligand 2tBuCzDPPZ. p2tBuCzDPPZRhCp* and 2TPADPPZRhCp* were not considered further, as p2tBuCzDPPZRhCp* features only a weak charge-transfer band in the UV-vis spectrum at ca. 600 nm with a low extinction coefficient (Fig. S54a), while for 2TPADPPZRhCp* initial photocatalysis tests revealed a low performance in NAD+ reduction (Fig. S54b).
Photophysics
We first investigated the optoelectronic properties of 2tBuCzDPPZ and 2tBuCzDPPZRhCp* to gain insight into their absorption characteristics and excited-state relaxation dynamics. Cyclic voltammetry (CV) in CH2Cl2 solution confirms a D–A character for 2tBuCzDPPZ with oxidation half-wave potentials of 0.74 V and 1.06 V (all redox potentials are given versus the ferrocene/ferrocenium redox couple (Fc/Fc+)) and a reduction half-wave potential of −1.70 V (Table 1 and SI, Fig. S25). The oxidations take place at the tert-butyl carbazoles,26 and the reduction occurs on the DPPZ core. In the PS-CAT dyad 2tBuCzDPPZRhCp*, the potentials are slightly shifted, and, in addition, new irreversible processes were observed at 1.13, −1.28, and −0.22 V. However, the wave at −0.22 V was only visible when both the oxidative and reductive areas were scanned. The two-electron reduction of the Rh center (RhIII/RhI) was observed at −1.21 V, which lies in the range of values reported in the literature.10,11 This indicates that after excitation the tert-butyl carbazoles can be re-reduced with common sacrificial electron donors (SEDs), since the oxidation potential of the SED is lower (e.g. Eox (NEt3): 0.31 V vs. Fc/Fc+)27 than E1/2-ox of 2tBuCzDPPZRhCp*.| Compound | E 1/2-ox vs. Fc/Fc+/V | Peak-to-peak separationa/V | E 1/2-red vs. Fc/Fc+/V | Peak-to-peak separationa/V |
|---|---|---|---|---|
| a Peak-to-peak separation for Fc/Fc+ in the measurement: 0.45 V (2tBuCzDPPZ) and 0.35 V (2tBuCzDPPZRhCp*). b From the literature-reported value of 1.12 V vs. SCE, 0.38 V was subtracted to obtain the value vs. Fc/Fc+.29 c Irreversible processes. d Anodic peak potential. e Cathodic peak potential. f Quasi-reversible. | ||||
| t BuCz26 | 0.78b | — | — | — |
| DPPZ28 | — | — | −1.6 | — |
| −2.06 | ||||
| 2tBuCzDPPZ | 0.74 | 0.31 | −1.70 | 0.19 |
| 1.06 | 0.16 | |||
| 2tBuCzDPPZRhCp* | 0.81 | 0.09 | −0.22c,e | 0.21 |
| 1.13c,d | — | −1.21 | 0.40f | |
| 1.21 | 0.73 | −1.28c,e | — | |
| −1.73 | — | |||
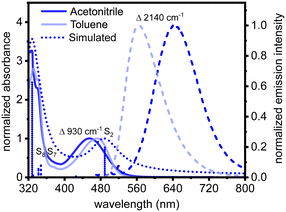
The ground-state absorption spectrum of 2tBuCzDPPZ in toluene features an absorption band centered at 472 nm (Fig. 2). Quantum chemical calculations associate this absorption feature with a dipole-allowed intramolecular charge transfer excitation of  character into the S2 state with an excitation wavelength of 499 nm (2.47 eV, Table S7). This band undergoes a hypsochromic shift to 452 nm in acetonitrile (Δ = −930 cm−1) without altering the character of the underlying electronic transition (S2 at 490 nm, 2.54 eV, Table S3). Notably, the transition into the S1 charge transfer state is dipole-forbidden (i.e. optically inactive) in both solvents. The emission maximum shifts from 563 nm in toluene to 645 nm in acetonitrile (Δ = 2140 cm−1). Such a red shift in a polar solvent is characteristic for CT emitters and suggests a stabilization of the lowest excited singlet state (S1) due to its strong dipole moment.30,31
character into the S2 state with an excitation wavelength of 499 nm (2.47 eV, Table S7). This band undergoes a hypsochromic shift to 452 nm in acetonitrile (Δ = −930 cm−1) without altering the character of the underlying electronic transition (S2 at 490 nm, 2.54 eV, Table S3). Notably, the transition into the S1 charge transfer state is dipole-forbidden (i.e. optically inactive) in both solvents. The emission maximum shifts from 563 nm in toluene to 645 nm in acetonitrile (Δ = 2140 cm−1). Such a red shift in a polar solvent is characteristic for CT emitters and suggests a stabilization of the lowest excited singlet state (S1) due to its strong dipole moment.30,31
A comparison of the fluorescence and phosphorescence spectra (recorded at 77 K in methylcyclohexane) provides a relatively small energy gap (ΔEST = 0.15 eV) between the first excited singlet and triplet states (Fig. S34 and Table S1). According to quantum chemical calculations at the Franck–Condon point, the vertical S1–T1 energy gaps are 0.25 and 0.26 eV in toluene and acetonitrile, respectively. In the case of toluene, the energy gap between the fully relaxed S1 and T1 states was predicted to be 0.25 eV, i.e. identical to the ΔEST in the Franck–Condon geometry (S0 equilibrium structure). Therefore, the subsequently discussed singlet-triplet energy gaps are exclusively obtained based on the vertical energy difference in the relaxed singlet ground state. Calculated rates of intersystem crossing (ISC) (kISC, SI, eqn (S1)) between lower lying singlet (S1 and S2) and triplet (T1 and T2) states, as well as rates of spontaneous emission (kF, SI, eqn (2)) were obtained in both acetonitrile and toluene and indicated rather slow and inefficient population transfer among low-lying singlet and triplet states as well as slow (spontaneous) emission based on the quasi dipole-forbidden S1 → S0 transition (Table S2).
The photoluminescence quantum yield (PLQY) of 2tBuCzDPPZ is strongly solvent-dependent, as typically observed for CT-emitters, where the excited CT state is stabilized in more polar solvents (Table 2): whereas in toluene the PLQY amounts to 36%, in acetonitrile it is only 2% (both argon-purged). In non-argon-purged (aerated) solvents the PLQYs are slightly lower (29% in toluene and 1% in acetonitrile). A strong increase in PLQY upon argon-purging of a dye solution typically indicates participation of a triplet excited state. Furthermore, a strong solvent (polarity) effect on PLQYs and on the excited-state properties is typical for photophysical properties of DPPZ-like scaffolds.53,54
| 2tBuCzDPPZ | 2tBuCzDPPZRhCp* | |||
|---|---|---|---|---|
| Air-saturated | Argon-purged | Air-saturated | Argon-purged | |
| a Bi-exponential decay. b Mono-exponential decay. | ||||
| PLQY/% (acetonitrile) | 1 | 2 | 1 | 1 |
| PLQY/% (toluene) | 29 | 36 | 10 | 12 |
| τ/ns (acetonitrile) | 0.10, 3.87a | 0.10, 4.87a | 0.28, 1.42a | 0.34, 1.91a |
| τ/ns (toluene) | 8.63b | 9.74b | 0.09, 5.34a | 0.15, 8.74a |
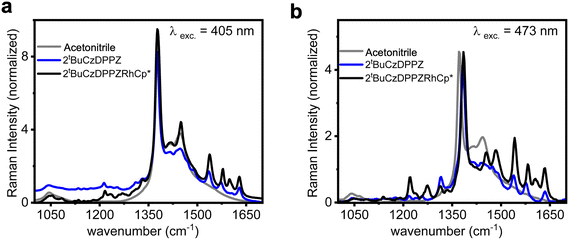
To further characterize the Franck–Condon point of 2tBuCzDPPZ and 2tBuCzDPPZRhCp*, the resonance Raman (rR) spectra of both compounds were measured in their acetonitrile solution. The rR selectively enhances vibrations of the ligands that are involved in the electronic transitions, and enables an understanding of the localization of the initially excited state of the chromophore. The rR spectra recorded upon 405 nm excitation are shown in Fig. 3. Both for 2tBuCzDPPZ and 2tBuCzDPPZRhCp* the Raman spectrum is dominated by sharp bands at 1536, 1577, 1602, and 1630 cm−1, along with a shoulder at 1487 cm−1 (Fig. 3a). These features are attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibrations of the DPPZ bridging ligand, indicating π–π* or intramolecular CT transitions centered on the DPPZ chromophore.32,33 Carbazole contributions at this excitation energy are minimal. Upon 473 nm excitation, a broader set of vibrational features emerges, including DPPZ-associated modes at 1541, 1582, 1606, and 1633 cm−1.34 Finally, the appearance of weak bands at 1219, 1271, 1315, 1455, and 1486 cm−1 points to the involvement of the carbazole moieties.35,36 The latter spectral pattern aligns well with Raman bands of free carbazole in dichloromethane, which are assigned to the in-plane bending and C–N/C
N stretching vibrations of the DPPZ bridging ligand, indicating π–π* or intramolecular CT transitions centered on the DPPZ chromophore.32,33 Carbazole contributions at this excitation energy are minimal. Upon 473 nm excitation, a broader set of vibrational features emerges, including DPPZ-associated modes at 1541, 1582, 1606, and 1633 cm−1.34 Finally, the appearance of weak bands at 1219, 1271, 1315, 1455, and 1486 cm−1 points to the involvement of the carbazole moieties.35,36 The latter spectral pattern aligns well with Raman bands of free carbazole in dichloromethane, which are assigned to the in-plane bending and C–N/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching modes of the carbazole framework.35,37,38 This suggests that 405 nm excitation leads to CT transitions (from the carbazoles to DPPZ). Upon longer-wavelength excitation at 473 nm, the intensity ratio of carbazole with the DPPZ ligand increases as compared to that at 405 nm (Fig. 3b; the Raman bands were normalized with respect to their absorption coefficient). The enhanced intensity of carbazole-based vibrational modes under 473 nm excitation suggests that this excitation involves the π-systems of both the terminal carbazole and the central DPPZ ligand. Overall, these results demonstrate that 405 nm excitation selectively enhances DPPZ-localized vibrations, while 473 nm excitation activates modes from both DPPZ and carbazole.
C stretching modes of the carbazole framework.35,37,38 This suggests that 405 nm excitation leads to CT transitions (from the carbazoles to DPPZ). Upon longer-wavelength excitation at 473 nm, the intensity ratio of carbazole with the DPPZ ligand increases as compared to that at 405 nm (Fig. 3b; the Raman bands were normalized with respect to their absorption coefficient). The enhanced intensity of carbazole-based vibrational modes under 473 nm excitation suggests that this excitation involves the π-systems of both the terminal carbazole and the central DPPZ ligand. Overall, these results demonstrate that 405 nm excitation selectively enhances DPPZ-localized vibrations, while 473 nm excitation activates modes from both DPPZ and carbazole.
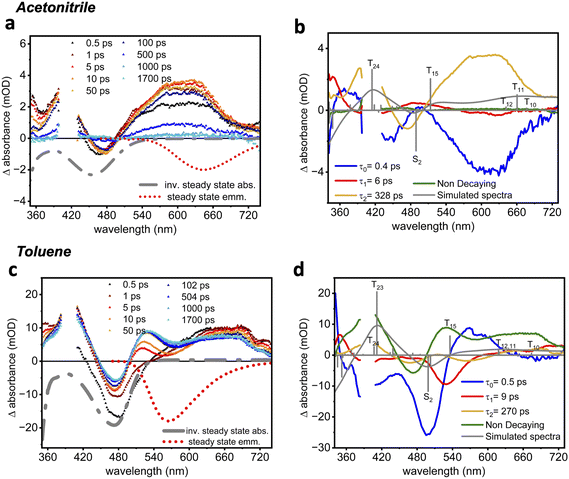
To gain insights into the excited-state dynamics of 2tBuCzDPPZ, femtosecond transient absorption (fs-TA) measurements were performed upon excitation at 400 nm (Fig. 4a). In acetonitrile, a pronounced ground-state bleach (GSB) is observed at 450 nm, consistent with the inverse of the steady-state absorption spectrum (Fig. 2a). In parallel, excited-state absorption (ESA) bands emerge at both short (360–400 nm) and long (>500 nm) probe wavelengths (Fig. S26 and S27). The long-wavelength ESA is assigned to an intramolecular CT transition from one carbazole unit to the phenazine core. Upon photoexcitation, both ESA bands rise rapidly, i.e. within the first 0.5 ps to 50 ps, followed by a decay onsetting after approximately 100 ps. As a result of this decay, approximately 95% of the initial excited-state population decays within 1 ns. The lifetime associated with the sub-ns decay is reminiscent of the fast, 0.1 to few ns component in the emission lifetime measurements (see Table 2).
Global kinetic analysis was performed using three decay components in addition to a non-decaying (within our experimental window of 1.8 ns) component to reasonably fit the TA signal (see Fig. 4). The resulting decay-associated spectra (DAS, Fig. 4b and d) show that the first two components, associated with the decay time constants τ0 = 0.4 ps and τ1 = 6 ps, correspond to ultrafast vibrational and electronic relaxations, respectively, within the initially populated 1CT state. According to DFT, the fully relaxed T1 state features a prominent CT character in acetonitrile which is associated with a charge redistribution from the π-system of the carbazole moieties towards the lowest energy  orbital. Excitation of this T1 state causes the ESA signal observed in the time-resolved measurements. More precisely, simulations of the TA spectra indicated that a 1
orbital. Excitation of this T1 state causes the ESA signal observed in the time-resolved measurements. More precisely, simulations of the TA spectra indicated that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 singlet-to-triplet ratio best accounts for the experimental data. According to TDA-TDDFT, the main contributions to the ESA stem from T24, T15, and T12–10, resulting in major absorption at ∼425 nm, ∼520 nm, and 630–680 nm, respectively (Fig. S49 and Table S6). Excitations into T10,11 and T24 feature mixed CT and locally excited characters, while T12 is of pure CT and T15 is of
3 singlet-to-triplet ratio best accounts for the experimental data. According to TDA-TDDFT, the main contributions to the ESA stem from T24, T15, and T12–10, resulting in major absorption at ∼425 nm, ∼520 nm, and 630–680 nm, respectively (Fig. S49 and Table S6). Excitations into T10,11 and T24 feature mixed CT and locally excited characters, while T12 is of pure CT and T15 is of  nature. Detailed information with respect to the electronic nature of the underlying transitions is collected and provided in the SI, as shown in Table S1. From this we assign the 328 ps component (τ2) to the decay of a long-lived 3CT state, while the long-lived non-decaying component is nearly featureless and may represent a non-decaying offset or minor contribution from a weakly emissive state.
nature. Detailed information with respect to the electronic nature of the underlying transitions is collected and provided in the SI, as shown in Table S1. From this we assign the 328 ps component (τ2) to the decay of a long-lived 3CT state, while the long-lived non-decaying component is nearly featureless and may represent a non-decaying offset or minor contribution from a weakly emissive state.
2tBuCzDPPZ in the nonpolar solvent toluene does not show the same results (Fig. 4d): within the first few picoseconds, an ESA band centered at around 650 nm is observed, mirroring the early-time spectral feature in acetonitrile. However, at later delay times (after ca. 5 ps), the ESA feature at 520 nm progressively intensifies, while the ESA at 620 nm decays. This behavior leads to the appearance of an isosbestic point at 590 nm, which indicates internal conversion between two states, assigned to simulated T11 and T15 states (Table S10). According to TDA-TDDFT, the nature of the transition into T11 in toluene shifts from partial CT in acetonitrile to purely CT in toluene, while the respective transition dipole moment decreases significantly. In contrast, the CT nature of T15 remains unaffected. These solvent-dependent relaxation channels – as evident by means of the difference in the ESA spectral profile – highlight the influence of solvent polarity on the excited-state dynamics, especially impacting the triplet state behavior and interconversion of the involved CT states.
We next investigated the photophysical properties of the PS-CAT dyad 2tBuCzDPPZRhCp* in more detail. Upon coordination of an Rh center to form the dyad 2tBuCzDPPZRhCp*, the intramolecular charge-transfer absorption further red-shifts to 492 nm compared to 2tBuCzDPPZ, independent of solvent polarity (Fig. 5a). According to the TDA-DFT calculations with the range-separated ωB97x-d3 functional as well as using the PBE0 global hybrid functional, this band can be consistently associated with a dipole allowed CT  transition into the S2, state (Table S1). However, the excitation energies are over- and underestimated, respectively, depending on the functional used, thus a further assignment of the subsequent excited-state processes in the PS-CAT dyad based on quantum chemical simulations was – unfortunately – not possible. The emission maxima of 2tBuCzDPPZRhCp* are observed at 647 nm in toluene and at 672 nm in acetonitrile, which correspond to only a small bathochromic shift in the more polar solvent (Δ = 575 cm−1). This red shift is three times smaller than that in 2tBuCzDPPZ, indicating a notable impact of the Rh center on the excited states associated with the phenazine core.
transition into the S2, state (Table S1). However, the excitation energies are over- and underestimated, respectively, depending on the functional used, thus a further assignment of the subsequent excited-state processes in the PS-CAT dyad based on quantum chemical simulations was – unfortunately – not possible. The emission maxima of 2tBuCzDPPZRhCp* are observed at 647 nm in toluene and at 672 nm in acetonitrile, which correspond to only a small bathochromic shift in the more polar solvent (Δ = 575 cm−1). This red shift is three times smaller than that in 2tBuCzDPPZ, indicating a notable impact of the Rh center on the excited states associated with the phenazine core.
Notably, the introduction of the Rh center significantly decreases the PLQY compared to the photosensitizer 2tBuCzDPPZ (Table 2). The PLQY drops from 36% (2tBuCzDPPZ) to 12% (2tBuCzDPPZRhCp*) in toluene (under an argon atmosphere), and from 2% to 1% in acetonitrile. This trend is consistent with the observed emission lifetimes (see SI Fig. S36 and S37). In toluene, the lifetime decreases from 9.7 ns (2tBuCzDPPZ) to 8.7 ns (2tBuCzDPPZRhCp*), whereas in acetonitrile it decreases from 4.9 ns (2tBuCzDPPZ) to 1.9 ns (2tBuCzDPPZRhCp*). These experimental observations point towards excited-state electron transfer from the photosensitizer to the Rh center and are corroborated by calculations of the non-radiative decay rates (knr). In toluene, the non-radiative rate increases from 6.6 × 107 s−1 for 2tBuCzDPPZ to 1.0 × 108 s−1 for 2tBuCzDPPZRhCp*—an approximate 50% increase. This effect is even more pronounced in acetonitrile, where knr dramatically increases from 2.0 × 108 s−1 (2tBuCzDPPZ) to 5.2 × 108 s−1 (2tBuCzDPPZRhCp*). These values for knr reflect solvent effects and reveal an increase in non-radiative decay pathways, potentially mediated by the coordinated Rh center. A similar effect has been documented in ruthenium and perylene dyads, and it can be rationalized by the transfer of excited-state electrons to the Rh metal center7,11 – a prerequisite for photocatalysis, during which the Rh(III) center must undergo a twofold reduction via PET to a Rh–H species, which then reduces NAD+ (see further below).
Femtosecond TA measurements on the dyad 2tBuCzDPPZRhCp* were performed both in acetonitrile and toluene (Fig. 5b, c and SI S31, S33). Upon coordination with the Rh center, the transient spectral features in acetonitrile resemble those of the PS 2tBuCzDPPZ, including a characteristic GSB at 505 nm and ESA bands in the regions of 360–400 nm and >500 nm (Fig. 5b). However, in acetonitrile the excited state of 2tBuCzDPPZRhCp* decays faster than that of 2tBuCzDPPZ (cf.Fig. 4a): the transient signals of 2tBuCzDPPZRhCp* decay almost completely (to 5% of the initial amplitude) within 92 ps. This stands in stark contrast to the comparably slow excited-state decay of 2tBuCzDPPZ. The rapid deactivation in 2tBuCzDPPZRhCp* is attributed to an accelerated photoinduced energy transfer (PEnT) facilitated by the Rh center. This behavior is consistent with prior studies showing enhanced PEnT in heavy-atom-containing systems.39–41 Interestingly, in toluene, PEnT seems to be prolonged, with ESA features persisting beyond our experimental window of 2 ns (Fig. 5c). Coordination of Rh significantly alters the excited-state dynamics of the 2tBuCzDPPZ photosensitizer. While the free PS exhibits two distinct ESA bands in toluene—indicative of internal conversion between triplet excited states (as shown in Fig. 4e)—2tBuCzDPPZRhCp* displays only a single ESA feature centered at 650 nm (Fig. 5c). This suggests that coordination to Rh simplifies the excited-state manifold so that only a single triplet state remains visible in the TA data. The complete recovery of the ground-state bleach within 1 ns in both solvents further supports the notion that the excited-state population fully relaxes back to the ground state. The polarization dependence of this process and the fact that such fast ground-state recovery is observed upon coordination of the Rh center suggest that the molecular origin of the excited-state relaxation is a PEnT towards the Rh(III) metal center. This has also been supported by the emission lifetime (see Table 2), where the introduction of Rh significantly reduces the emission lifetime of the 2tBuCzDPPZ chromophore.
Photocatalytic performance of the PS-CAT dyad
We next set out to investigate the photocatalytic performance of the new PS-CAT dyad. Prior to photocatalysis we checked the general activity of the catalytic Rh(III)Cp* center in 2tBuCzDPPZRhCp* for NAD+ reduction in a “thermal” (dark) catalysis.42,43 For this, sodium formate was used as the reducing agent. It is anticipated that the dye center (PS) has no influence on the outcome of the thermal catalysis, as reported by Zedler and co-workers.17,41,44 They observed a similar performance for three PSs with different bridging ligands to the Rh metal center and a strong temperature dependence in accordance with an Arrhenius plot since the reaction only involved the Rh-metal center and not the bridging ligand or the PS part. In our case we could observe catalytic activity (TON: 8 (after 60 min, 45 °C), TOF: 21 h−1 (after 5 min, 45 °C), see SI Fig. S42) under similar conditions, which demonstrates that 2tBuCzDPPZRhCp* is in principle capable of reducing NAD+ to NADH. However, in the literature higher TONs and TOFs were achieved by using different catalysts (e.g. RutpphzRhCp*: TON: 43 (after 50 min, 45 °C), TOF: 75 h−1 (after 5 min, 45 °C)).41 One explanation for this might be the slightly more negative redox potential (100 mV) for the Rh(III)/Rh(I) couple of the 2tBuCzDPPZRhCp* dyad in comparison to the Ru-based dyads.41 A more negative potential is associated with a lower NAD+ reduction rate.43 In addition, it has been previously shown for DPPZ-containing Ru(II) polypyridine complexes that NADH is strongly stacking to the DPPZ moiety of the complex.45 This could potentially lead to it being readily available for reoxidation of NADH into NAD+ by Rh(III).To assess the photocatalytic activity of the new PS-CAT dyad we studied the light-driven reduction of NAD+ to NADH. The mechanism of this process starts by exciting 2tBuCzDPPZRhCp* with LED light (λmax = 465 nm, fwhm = 20 nm, P = 45 mW cm−2), which is assumed to cause a photo-induced electron transfer from the PS to the Rh center (Fig. 6a). This process must occur twice, as the Rh(III) has to be reduced to Rh(I) under the loss of an anionic ligand. To regenerate the oxidized carbazole, a sacrificial electron donor (SED) is needed. Oftentimes triethylamine (TEA) fulfills the intended purpose, although it must be used in high quantities (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 eq. relative to the 2tBuCzDPPZRhCp* dyad).10,11 After the oxidative addition of a proton to the Rh center (red in Fig. 6a), the final hydride transfer from the latter to NAD+ can take place to afford NADH.10
000 eq. relative to the 2tBuCzDPPZRhCp* dyad).10,11 After the oxidative addition of a proton to the Rh center (red in Fig. 6a), the final hydride transfer from the latter to NAD+ can take place to afford NADH.10
 | ||
| Fig. 6 (a) Proposed mechanism of the light-driven NAD+ reduction using the PS-CAT dyad 2tBuCzDPPZRhCp*.10 (b) UV/vis spectra of the catalytic mixture of dyad 2tBuCzDPPZRhCp* (5 µM) with TEA (0.212 M) as an SED (0.17 M NaH2PO4, LED (λmax = 465 nm, fwhm = 20 nm, P = 45 mW cm−2), acetonitrile/water (1/2, v/v)). (c) Mechanism of BIH serving as the SED.27 (d) Formation of NADH monitored by fluorescence spectroscopy with (e) TON and TOF values under the following conditions in acetonitrile/water (1/2, v/v): 5 µM 2tBuCzDPPZRhCp*, 400 µM BIH-OMe, 250 µM NAD+, LED (λmax = 465 nm, fwhm = 20 nm, P = 45 mW cm−2); dark reaction: 5 µM 2tBuCzDPPZRhCp*, 400 µM BIH-OMe, 250 µM NAD+; no catalyst: 400 µM BIH-OMe, 250 µM NAD+, LED (λmax = 465 nm, fwhm = 20 nm, P = 45 mW cm−2). | ||
In our first experiments we tested the well-established conditions with TEA (pKa (HNEt3+): 10.76; Eox (NEt3): 0.31 V vs. Fc/Fc+)27 as an SED as its redox potential fits for reducing the oxidized carbazoles (cf.Table 1).10,11 However, with the 2tBuCzDPPZRhCp* dyad, no NADH was formed under these conditions. In situ spectroscopic investigations revealed that during photocatalysis a broad absorption band at 666 nm arises within minutes (Fig. 6b). This can be attributed to the formation of an Rh(I) species, which is an intermediate in the catalytic cycle (Fig. 6a). The formation of this intermediate in a TEA/phosphate buffer could previously only be observed when no NAD+ was present but is typically found if dihydrogenphosphate is left out of the solution.10,11,20 Its accumulation indicates that the next step of the catalytic cycle is hindered, the oxidative addition of a proton. From this, one can conclude that the pKa value of the Rh–H species is lower than the pKa value of the buffer solution. Increasing the buffer concentration did not solve this problem. Shifting to other amine-based SEDs with lower pKa values was also not successful. Triethanolamine (pKa (HN(CH2CH2OH)3+): 7.74; Eox (N(CH2CH2OH)3): 0.19–0.44 vs. Fc/Fc+)27,46 performed similar to TEA, and the aromatic amines dimethylaniline (pKa (HNMe2Ph+): 5.15; Eox (NMe2Ph): 0.43 vs. Fc/Fc+)27,47 and N,N-dimethyl-p-toluidine (pKa (HNMe2(C6H4Me)+): 5.63; Eox (NMe2(C6H4Me)): 0.33 vs. Fc/Fc+)27,48 showed neither NADH nor Rh(I) formation. Finally, modified benzimidazole SEDs brought success. 1,3-Dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole (BIH) is well known both as a hydride donor and as an SED (Fig. 6c).27 BIH and its derivatives were for instance employed by Ishitani as an SED for the HER and CO2 reduction with Ru-RhCp* dyads.49,50 The mechanism of action when BIH serves as an SED is shown in Fig. 6c and involves a stepwise oxidation via successive one-electron transfer processes as well as an associated deprotonation.27 BIH therefore supplies everything needed for the NAD+ reduction. Initial tests with this SED showed promising results, although BIH was almost insoluble in the catalytic mixture.
To increase solubility, we attached a methoxy group to the phenyl substituent of BIH, resulting in BIH-OMe.51 Being sufficiently soluble in the acetonitrile/water mixture used in photocatalysis, BIH-OMe was finally able to serve as a functional SED in the light-driven NAD+ reduction with dyad 2tBuCzDPPZRhCp*. The amount of NADH was determined by fluorescence spectroscopy (Fig. 6d, λmax(NADH): 461.5 nm, see SI Fig. S38 and S39). Under the optimized conditions dyad 2tBuCzDPPZRhCp* delivered NADH with a TON of 3.2 after 4 h and a maximum TOF of 1.2 h−1 (after 10 min) (Fig. 6e). For the literature-known Ru-based dyad RutpphzRhCp*, TONs up to 8 (after 60 min) and TOFs of 9 h−1 (after 60 min) could be observed.41 In the case of the perylene-based dyad, TONs of 4 and TOFs of 4 h−1 (after 60 min) were reported.11 Despite the lower values of the herein reported dyad, it could be shown that 2tBuCzDPPZRhCp* works as a proof-of-concept D–A-PS-CAT dyad, and many design alterations are possible to improve performance in the future.
Control experiments with no catalyst and no LED irradiation demonstrated that all components are crucial to form NADH (Fig. 6e). Prior to catalysis, BIH-OMe was also tested in emission quenching experiments of the PS 2tBuCzDPPZ, which resulted in a bimolecular quenching constant of kq-I = 5.06 × 109 M−1 s−1 for emission quenching, and a bimolecular quenching constant of kq-τ = 7.46 × 109 M−1 s−1 for lifetime quenching (see SI Fig. S35). This is close to the diffusion-controlled limit of the quenching rate, which is ∼1 × 1010 M−1 s−1 (ref. 52) and confirms that electron transfer from BIH-OMe to the CT-excited state of the PS 2tBuCzDPPZ is a rapid event.
It is worth noting that BIH-OMe already performs well at a relatively low quantity of 80 equivalents of the PS-CAT dyad. When increasing the concentration from 250 µM to 400 µM, significantly more NADH is produced over time. However, beyond this point (3300 µM of BIH-OMe), no further NADH is formed, but the catalyst starts degrading (see SI Fig. S47). In comparison to photocatalysis with other dyads using 12 mM TEA as an SED,10 in our case 300-fold lower SED concentration is needed to run the reaction, meaning that less chemical waste is produced.
On performing the light-driven catalysis at a higher temperature, emission bands in the range from 370 to 385 nm can be observed, which can be attributed to a degradation product of BIH-OMe (SI, Fig. S46). These bands increase in intensity with the irradiation time. This indicates that BIH-OMe undergoes some kind of side reaction, which is not coupled with the actual catalysis. This side reaction becomes dominant at higher temperatures making BIH-OMe not suitable as an SED under these conditions.
Conclusions
We herein present the donor–acceptor design of the photosensitizer-catalyst (PS-CAT) dyad 2tBuCzDPPZRhCp* for nicotinamide hydrogenation/reduction. PS-CAT dyads mimic part of nature's complex assembly active in photosynthesis. The organic PS part with a donor (carbazole)-acceptor (dipyrido[3,2-a:2,3-c]phenazine = DPPZ) structure and substantial visible-light absorption contains a bidentate coordination site for a catalytically active rhodium(III) center. The presented derivatives of this principal building block all show photoluminescence with lifetimes of 10 ns and 36% photoluminescence quantum yield. Photophysical investigations on the PS 2tBuCzDPPZ, including femtosecond transient absorption (fs-TA) measurements and supported by TDDFT calculations, revealed a strong solvent (polarity) effect on photoluminescence quantum yields and on the excited-state properties. In addition, we postulate that a fast excited-state energy transfer from the 2tBuCzDPPZ PS to the Rh center takes place. This can be considered as a deactivation pathway of the crucially important excited state and might be a reason for the low photocatalytic activity. The PS-CAT dyad was employed in the light-driven NAD+ to NADH reduction and showed – after identification of BIH-OMe as a suitable sacrificial electron donor (SED) – photocatalytic performance with a TON of 3.2 (after 4 h) and a TOF of up to 1.2 h−1. The fact that only a strong SED resulted in catalytic turnover is an indication that the lifetime of the charge-separated state is short and therefore limits the overall photocatalytic potential. This study provides a new approach to PS-CAT dyads using organic D–A chromophores as a PS, stimulating the future development of new types of organic PS-based dyads. A future pathway for the optimization opened by this combined synthetic, theoretic and spectroscopic investigation is to close the energy transfer pathway between the charge-separated state and the Rh(III)Cp* center, to favor the required electron transfer pathway.Author contributions
Conceptualization: B. E., A. T., and B. D.-I.; data curation: all authors; formal analysis: A. T., M. S., and T. M.; funding acquisition: B. E., B. D.-I., S. K., S. R., and A. J. C. K.; investigation: A. T., M. S., T. M., and D. S.; methodology: all authors; project administration: B. E., B. D.-I., and S. K.; resources: B. E., B. D.-I., S. K., S. R., and A. J. C. K.; supervision: B. E., B. D.-I., and S. K.; validation: all authors; visualization: A. T., M. S., T. M., and B. E.; writing – original draft: A. T. and B. E.; writing – review & editing: all authors.Conflicts of interest
There are no conflicts to declare.Data availability
The data obtained in this study have been deposited in the repository Zenodo and are available under the digital object identifier: https://doi.org/10.5281/zenodo.17493527.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5sc08675b.
Acknowledgements
The authors thank Andrea Pannwitz (Friedrich Schiller University of Jena) and Bernhard Putz (Ulm University) for help with TCSPC measurements and Linda Zedler (Leibniz-Institute of Photonic Technology) for assistance with resonance Raman experiments. This work was funded by the German Research Foundation (DFG, German Research Foundation) under project IDs 364549901 (SFB TRR 234 “CataLight” (projects A1, A4, A6)), 445471845, 445471097, 456129967, and 445470598.References
- W. W. Fischer, J. Hemp and J. E. Johnson, Evolution of Oxygenic Photosynthesis, Annu. Rev. Earth Planet. Sci., 2016, 44, 647–683 CrossRef CAS.
- T. S. Teets and D. G. Nocera, Photocatalytic hydrogen production, Chem. Commun., 2011, 47, 9268 RSC.
- M. Schmidt and B. Esser, Cavity-promotion by pillar[5]arenes expedites organic photoredox-catalysed reductive dehalogenations, Chem. Commun., 2021, 57, 9582–9585 RSC.
- K. L. Mulfort and L. M. Utschig, Modular Homogeneous Chromophore–Catalyst Assemblies, Acc. Chem. Res., 2016, 49, 835–843 CrossRef CAS PubMed.
- M. G. Pfeffer, T. Kowacs, M. Wächtler, J. Guthmuller, B. Dietzek, J. G. Vos and S. Rau, Optimization of Hydrogen-Evolving Photochemical Molecular Devices, Angew. Chem., Int. Ed., 2015, 54, 6627–6631 Search PubMed.
- D. Costabel, R. De, F. Jacobi, J. Eichhorn, K. Hotzel, A. Nabiyan, C. Neumann, A. Turchanin, S. Kupfer, F. H. Schacher, S. Rau, B. Dietzek-Ivanšić and K. Peneva, Bridging Platinum and Palladium to Bipyridine-Annulated Perylene for Light-Driven Hydrogen Evolution, ACS Catal., 2023, 13, 7159–7169 Search PubMed.
- A. K. Mengele, S. Kaufhold, C. Streb and S. Rau, Generation of a stable supramolecular hydrogen evolving photocatalyst by alteration of the catalytic center, Dalton Trans., 2016, 45, 6612–6618 Search PubMed.
- W. Paw, W. B. Connick and R. Eisenberg, Dipyridocatecholate-Bridged Complexes of Platinum and Ruthenium Diimine Chromophores, Inorg. Chem., 1998, 37, 3919–3926 Search PubMed.
- S. Rau, B. Schäfer, D. Gleich, E. Anders, M. Rudolph, M. Friedrich, H. Görls, W. Henry and J. G. Vos, A Supramolecular Photocatalyst for the Production of Hydrogen and the Selective Hydrogenation of Tolane, Angew. Chem., Int. Ed., 2006, 45, 6215–6218 Search PubMed.
- L. Zedler, P. Wintergerst, A. K. Mengele, C. Müller, C. Li, B. Dietzek-Ivanšić and S. Rau, Outpacing conventional nicotinamide hydrogenation catalysis by a strongly communicating heterodinuclear photocatalyst, Nat. Commun., 2022, 13, 2538 CrossRef CAS.
- J. Brückmann, C. Müller, I. Friedländer, A. K. Mengele, K. Peneva, B. Dietzek-Ivansic and S. Rau, Photocatalytic Reduction of Nicotinamide Co-factor by Perylene Sensitized RhII IComplexes, Chem.–Eur. J., 2022, 23, e202201931 CrossRef PubMed.
- P. Buday, C. Kasahara, E. Hofmeister, D. Kowalczyk, M. K. Farh, S. Riediger, M. Schulz, M. Wächtler, S. Furukawa, M. Saito, D. Ziegenbalg, S. Gräfe, P. Bäuerle, S. Kupfer, B. Dietzek-Ivanšić and W. Weigand, Activating a [FeFe] Hydrogenase Mimic for Hydrogen Evolution under Visible Light, Angew. Chem., Int. Ed., 2022, 61, e202202079 CrossRef CAS PubMed.
- G.-G. Luo, K. Fang, J.-H. Wu, J.-C. Dai and Q.-H. Zhao, Noble-metal-free BODIPY–cobaloxime photocatalysts for visible-light-driven hydrogen production, Phys. Chem. Chem. Phys., 2014, 16, 23884–23894 Search PubMed.
- B. S. Veldkamp, W.-S. Han, S. M. Dyar, S. W. Eaton, M. A. Ratner and M. R. Wasielewski, Photoinitiated multi-step charge separation and ultrafast charge transfer induced dissociation in a pyridyl-linked photosensitizer–cobaloxime assembly, Energy Environ. Sci., 2013, 6, 1917 Search PubMed.
- M. B. Chambers, X. Wang, N. Elgrishi, C. H. Hendon, A. Walsh, J. Bonnefoy, J. Canivet, E. A. Quadrelli, D. Farrusseng, C. Mellot-Draznieks and M. Fontecave, Photocatalytic Carbon Dioxide Reduction with Rhodium-based Catalysts in Solution and Heterogenized within Metal–Organic Frameworks, ChemSusChem, 2015, 8, 603–608 CrossRef CAS PubMed.
- V. Ganesan, J. J. Kim, J. Shin, K. Park and S. Yoon, Efficient Nicotinamide Adenine Dinucleotide Regeneration with a Rhodium–Carbene Catalyst and Isolation of a Hydride Intermediate, Inorg. Chem., 2022, 61, 5683–5690 CrossRef CAS PubMed.
- C. L. Pitman, O. N. L. Finster and A. J. M. Miller, Cyclopentadiene-mediated hydride transfer from rhodium complexes, Chem. Commun., 2016, 52, 9105–9108 Search PubMed.
- C. Park, C. Kwon and Y. H. Hong, Designing biomimetic catalytic systems for CO2 reduction to formate using NAD(P)H, Inorg. Chem. Front., 2025, 12, 4544–4568 RSC.
- E. J. Askins, A. Sarkar, P. Navabi, K. Kumar, S. J. Finkelmeyer, M. Presselt, J. Cabana and K. D. Glusac, Interfacial Electrochemistry of Catalyst-Coordinated Graphene Nanoribbons, J. Am. Chem. Soc., 2024, 146, 22360–22373 Search PubMed.
- L. Zedler, A. K. Mengele, K. M. Ziems, Y. Zhang, M. Wächtler, S. Gräfe, T. Pascher, S. Rau, S. Kupfer and B. Dietzek, Unraveling the Light-Activated Reaction Mechanism in a Catalytically Competent Key Intermediate of a Multifunctional Molecular Catalyst for Artificial Photosynthesis, Angew. Chem., Int. Ed., 2019, 58, 13140–13148 CrossRef CAS PubMed.
- G. E. Shillito, S. Rau and S. Kupfer, Plugging the 3MC Sink in RuII-Based Photocatalysts, ChemCatChem, 2023, 15, e202201489 Search PubMed.
- L. Morgan, G. Pavan, N. Demitri, C. Alberoni, T. Scattolin, M. Roverso, S. Bogialli and A. Aliprandi, Tailoring thermally activated delayed fluorescence emitters for efficient electrochemiluminescence with tripropylamine as coreactant, RSC Adv., 2023, 13, 34520–34523 RSC.
- S. Amthor, D. Hernández-Castillo, B. Maryasin, P. Seeber, A. K. Mengele, S. Gräfe, L. González and S. Rau, Strong Ligand Stabilization Based on π-Extension in a Series of Ruthenium Terpyridine Water Oxidation Catalysts, Chem.–Eur. J., 2021, 27, 16871–16878 CrossRef CAS PubMed.
- M. Lämmle, A. K. Mengele, G. E. Shillito, S. Kupfer and S. Rau, Stability of Catalytic Centres in Light-Driven Hydrogen Evolution by Di- and Oligonuclear Photocatalysts, Chem.–Eur. J., 2023, 29, e202202722 CrossRef PubMed.
- Y. Zhang, J. Zhang, C. Shi, N. Sun and Q. Wang, Dipyrido[3,2-a:2′,3′-c]phenazine acceptor based thermally activated delayed fluorescence emitters, Dyes Pigm., 2022, 206, 110634 Search PubMed.
- H. Zhang, S. Wang, Y. Li, B. Zhang, C. Du, X. Wan and Y. Chen, Synthesis, characterization, and electroluminescent properties of star shaped donor–acceptor dendrimers with carbazole dendrons as peripheral branches and heterotriangulene as central core, Tetrahedron, 2009, 65, 4455–4463 CrossRef CAS.
- Y. Pellegrin and F. Odobel, Sacrificial electron donor reagents for solar fuel production, C. R. Chim., 2017, 20, 283–295 CrossRef CAS.
- Y. Liang, M. T. Nguyen, B. J. Holliday and R. A. Jones, Electrocatalytic reduction of CO2 using rhenium complexes with dipyrido[3,2-a:2′,3′-c]phenazine ligands, Inorg. Chem. Commun., 2017, 84, 113–117 CrossRef CAS.
- V. V. Pavlishchuk and A. W. Addison, Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25°C, Inorg. Chim. Acta, 2000, 298, 97–102 Search PubMed.
- R. Ishimatsu, S. Matsunami, K. Shizu, C. Adachi, K. Nakano and T. Imato, Solvent Effect on Thermally Activated Delayed Fluorescence by 1,2,3,5-Tetrakis(carbazol-9-yl)-4,6-dicyanobenzene, J. Phys. Chem. A, 2013, 117, 5607–5612 Search PubMed.
- Y. Yang, Z. Jiang, Y. Liu, T. Guan, Q. Zhang, C. Qin, K. Jiang and Y. Liu, Transient Absorption Spectroscopy of a Carbazole-Based Room-Temperature Phosphorescent Molecule: Real-Time Monitoring of Singlet–Triplet Transitions, J. Phys. Chem. Lett., 2022, 13, 9381–9389 CrossRef CAS PubMed.
- J. R. Schoonover, W. D. Bates and T. J. Meyer, Application of Resonance Raman Spectroscopy to Electronic Structure in Metal Complex Excited States. Excited-State Ordering and Electron Delocalization in Dipyrido[3,2-a:2’,3’-c]phenazine (dppz): Complexes of Re(I) and Ru(II), Inorg. Chem., 1995, 34, 6421–6422 Search PubMed.
- W. R. Browne and J. J. McGarvey, Raman scattering and photophysics in spin-state-labile d6 metal complexes, Coord. Chem. Rev., 2006, 250, 1696–1709 Search PubMed.
- C. Kuhnt, M. Karnahl, S. Tschierlei, K. Griebenow, M. Schmitt, B. Schäfer, S. Krieck, H. Görls, S. Rau, B. Dietzek and J. Popp, Substitution-controlled ultrafast excited-state processes in Ru–dppz-derivatives, Phys. Chem. Chem. Phys., 2010, 12, 1357–1368 RSC.
- A. Bree and R. Zwarich, Vibrational Assignment of Carbazole from Infrared, Raman, and Fluorescence Spectra, J. Chem. Phys., 1968, 49, 3344–3355 Search PubMed.
- M. Wächtler, J. Guthmuller, L. González and B. Dietzek, Analysis and characterization of coordination compounds by resonance Raman spectroscopy, Coord. Chem. Rev., 2012, 256, 1479–1508 CrossRef.
- L. Zhao, P. Wagner, A. B. S. Elliott, M. J. Griffith, T. M. Clarke, K. C. Gordon, S. Mori and A. J. Mozer, Enhanced performance of dye-sensitized solar cells using carbazole-substituted di-chromophoric porphyrin dyes, J. Mater. Chem. A, 2014, 2, 16963–16977 RSC.
- L. Kortekaas, F. Lancia, J. D. Steen and W. R. Browne, Reversible Charge Trapping in Bis-Carbazole-Diimide Redox Polymers with Complete Luminescence Quenching Enabling Nondestructive Read-Out by Resonance Raman Spectroscopy, J. Phys. Chem. C, 2017, 121, 14688–14702 CrossRef CAS PubMed.
- J. K. McCusker, Femtosecond Absorption Spectroscopy of Transition Metal Charge-Transfer Complexes, Acc. Chem. Res., 2003, 36, 876–887 CrossRef CAS.
- J. G. Santaclara, F. Kapteijn, J. Gascon and M. A. van der Veen, Understanding metal–organic frameworks for photocatalytic solar fuel production, CrystEngComm, 2017, 19, 4118–4125 Search PubMed.
- L. Zedler, P. Wintergerst, A. K. Mengele, C. Müller, C. Li, B. Dietzek-Ivanšić and S. Rau, Outpacing conventional nicotinamide hydrogenation catalysis by a strongly communicating heterodinuclear photocatalyst, Nat. Commun., 2022, 13, 2538 CrossRef CAS PubMed.
- H. C. Lo, C. Leiva, O. Buriez, J. B. Kerr, M. M. Olmstead and R. H. Fish, Bioorganometallic Chemistry. 13. Regioselective Reduction of NAD+ Models, 1-Benzylnicotinamde Triflate and β-Nicotinamide Ribose-5‘-methyl Phosphate, with in Situ Generated [Cp*Rh(Bpy)H]+ : Structure–Activity Relationships, Kinetics, and Mechanistic Aspects in the Formation of the 1,4-NADH Derivatives, Inorg. Chem., 2001, 40, 6705–6716 CrossRef CAS PubMed.
- V. Ganesan, D. Sivanesan and S. Yoon, Correlation between the Structure and Catalytic Activity of [Cp*Rh(Substituted Bipyridine)] Complexes for NADH Regeneration, Inorg. Chem., 2017, 56, 1366–1374 Search PubMed.
- A. Marrone and R. H. Fish, DFT Mechanism Studies: Biomimetic 1,4-NADH Chemoselective, Co-factor Regeneration with [Cp*Rh(bpy)H]+, in Tandem with the Biocatalysis Pathways of a Core Model of the (HLADH)-Zn(II) Mediated Enzyme, in the Enantioselective Reduction of Achiral Ketones to Chiral S-Alcohols, J. Organomet. Chem., 2021, 943, 121810 Search PubMed.
- A. K. Mengele, D. Weixler, A. Chettri, M. Maurer, F. L. Huber, G. M. Seibold, B. Dietzek, B. J. Eikmanns and S. Rau, Switching the Mechanism of NADH Photooxidation by Supramolecular Interactions, Chem.–Eur. J., 2021, 27, 16840–16845 CrossRef CAS PubMed.
- M. R. Simond, K. Ballerat-Busserolles, Y. Coulier, L. Rodier and J.-Y. Coxam, Dissociation Constants of Protonated Amines in Water at Temperatures from 293.15 K to 343.15 K, J. Solution Chem., 2012, 41, 130–142 CrossRef CAS.
- H. P. Marshall and E. Grunwald, The Measurement and Correlation of Acid Dissociation Constants for Ammonium and Anilinium Salts in the System Dioxane-Water1, J. Am. Chem. Soc., 1954, 76, 2000–2004 CrossRef CAS.
- M. M. Fickling, A. Fischer, B. R. Mann, J. Packer and J. Vaughan, Hammett Substituent Constants for Electron-withdrawing Substituents: Dissociation of Phenols, Anilinium Ions and Dimethylanilinium Ions, J. Am. Chem. Soc., 1959, 81, 4226–4230 Search PubMed.
- D. Ghosh, D. C. Fabry, D. Saito and O. Ishitani, Photochemical H2 Evolution Using a Ru–Rh Supramolecular Photocatalyst, Energy Fuels, 2021, 35, 19069–19080 Search PubMed.
- D. Ghosh, H. Takeda, D. C. Fabry, Y. Tamaki and O. Ishitani, Supramolecular Photocatalyst with a Rh(III)-Complex Catalyst Unit for CO2 Reduction, ACS Sustainable Chem. Eng., 2019, 7, 2648–2657 CrossRef CAS.
- L. Gimeno, C. Queffelec, E. Blart and Y. Pellegrin, Copper(I) Bis(diimine) Complexes with High Photooxidation Power: Reductive Quenching of the Excited State with a Benzimidazoline Sacrificial Donor, ACS Omega, 2022, 7, 13112–13119 CrossRef CAS PubMed.
- P. Du, J. Schneider, G. Luo, W. W. Brennessel and R. Eisenberg, Visible Light-Driven Hydrogen Production from Aqueous Protons Catalyzed by Molecular Cobaloxime Catalysts, Inorg. Chem., 2009, 48, 4952–4962 CrossRef CAS PubMed.
- D. A. McGovern, A. Selmi, J. E. O'Brien, J. M. Kelly and C. Long, Reduction of dipyrido-[3,2-a:2′,3′-c]-phenazine (dppz) by photolysis in ethanol solution, Chem. Commun., 2005, 1402–1404 RSC.
- T. Phillips, C. Rajput, L. Twyman, I. Haq and J. A. Thomas, Water-soluble organic dppz analogues—tuning DNA binding affinities, luminescence, and photo-redox properties, Chem. Commun., 2005, 4327 RSC.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2026 |