Open Access Article
Open Access ArticleEnantioselective C(sp3)–H bond functionalization enabled by CpxM(III) catalysis (M = Co, Rh, Ir)
Shu-Bin
Mou†
ab,
Mu-Peng
Luo†
b,
Feifei
Fang†
b,
Shi
Cao
*b,
Dong
Wu
*a and
Shou-Guo
Wang
 *b
*b
aComputer Aided Drug Discovery Center, Zhuhai Institute of Advanced Technology, Chinese Academy of Sciences, Zhuhai 519003, P. R. China. E-mail: wudong@ziat.ac.cn
bCollege of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen 518060, P. R. China. E-mail: shicaorganic@szu.edu.cn; shouguo.wang@szu.edu.cn
First published on 21st January 2026
Abstract
Trivalent group 9 transition metals (Co, Rh, and Ir) bearing pentamethylcyclopentadienyl (Cpx) or structurally tailored Cpx ligands have emerged as a transformative platform for asymmetric C(sp3)–H functionalization. Despite significant progress in C(sp2)–H activation, achieving site- and stereoselective transformation of C(sp3)–H bonds remains a formidable synthetic challenge due to their intrinsic inertness, increased steric congestion and conformational flexibility. This review provides a comprehensive overview of recent advances in asymmetric C(sp3)–H functionalization catalyzed by CpxM(III) (M = Co, Rh, Ir) complexes. Particular emphasis is placed on the evolution of chiral ligand frameworks, the strategic use of directing groups, and mechanistic insights into enantioselectivity. By integrating recent developments, this review highlights novel reactions and effective catalytic systems, clarifies remaining challenges, and outlines future research directions in this dynamic field. We anticipate that more novel CpxM(III)-catalyzed asymmetric C(sp3)–H functionalization reactions will be developed and will find broad applicability in the near future.
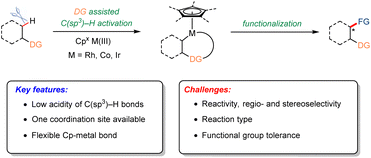
1 Introduction
Over the past twenty years, tremendous progress in catalyst design, ligand innovation, and mechanistic elucidation has transformed transition metal-catalyzed C–H functionalization into a broadly applicable paradigm for complex molecule construction.1–13 Transition metal-catalyzed C–H functionalization now provides a versatile and efficient platform for atom- and step-economical access to structurally diverse chiral molecules.14–19 A wide range of transition metals have been successfully employed in asymmetric C–H activation, with notable contributions from palladium,20–23 rhodium,24–27 iridium,28–31 cobalt32–35 and others.36–39 While the asymmetric functionalization of aromatic C(sp2)–H bonds has advanced rapidly,40–42 the direct enantioselective transformation of aliphatic C(sp3)–H bonds remains a formidable challenge, owing to their lower acidity (higher pKa), low polarity, and intrinsic inertness. Direct, selective, and enantioselective C(sp3)–H functionalization thus represents a streamlined strategy for installing stereogenic centers into simple hydrocarbons, enabling efficient construction of molecular complexity with profound implications for chiral molecule synthesis.Pioneering contributions from the Ward and Rovis groups,43 along with the Cramer group,44 have played a central role in shaping chiral CpxRh(III)-catalyzed asymmetric C–H functionalization. A particularly transformative advance has been the development of chiral cyclopentadienyl (Cpx) metal complexes, especially CpxM(III) catalysts based on cobalt, rhodium, and iridium.45–48 These catalysts combine exceptional reactivity with precise stereocontrol, enabling regio- and enantioselective formation of C–C and C–X bonds directly from inert aliphatic C–H sites, thereby eliminating the need for prefunctionalized substrates. The broad applicability of this approach—from pharmaceutical discovery to advanced material synthesis—further underscores its impact.26,49–52 The rapid evolution of chiral CpxM(III) catalysts, novel reaction methodologies, and mechanistic understanding has collectively propelled the field forward.24,27,32 These transformations typically proceed through substrate coordination to the metal center, selective C–H bond cleavage to generate an alkyl–metal intermediate, and subsequent functionalization (Scheme 1). The modular steric and electronic tunability of Cpx ligands provides fine control of both regio- and enantioselectivity, enabling the direct synthesis of enantioenriched molecules with high levels of asymmetric induction. Mechanistic studies have further revealed the critical roles of metal–ligand cooperativity, coordination geometry, and substrate stereoelectronics in governing reactivity and selectivity. As these catalytic systems mature, they are poised to become indispensable tools for stereoselective synthesis, transforming simple aliphatic hydrocarbons into architecturally sophisticated chiral molecules.
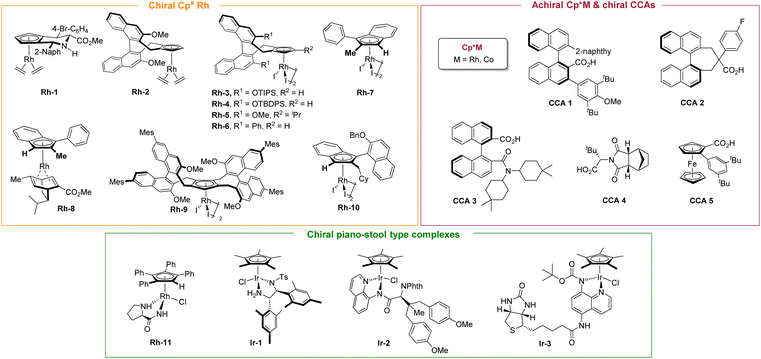
Enantiocontrol in C(sp3)–H functionalization has primarily been achieved through three complementary strategies that exploit distinct chiral sources to deliver high levels of stereoselectivity (Scheme 2). The most extensively developed approach employs chiral cyclopentadienyl (Cp or Cpx) metal(III) complexes, which provide a robust platform for C(sp3)–H activation.25,53 By tailoring the steric and electronic properties of the Cp framework, a highly organized chiral environment is established around the metal center, enabling precise enantioselective bond formation. A second strategy uses achiral CpxM(III) complexes in combination with chiral carboxylic acid (CCA) additives.49 In this co-catalytic system, the CCA functions as a transient chiral ligand or proton shuttle during the concerted metalation-deprotonation (CMD) step, transferring stereochemical information through a dynamic anion–metal interaction. This approach is operationally simple, highly modular, and particularly effective for challenging substrates. A third, conceptually distinct avenue employs chiral piano-stool complexes that operate via outer-sphere mechanisms.54–56 Here, stereocontrol arises not from direct metal–substrate bonding but from non-covalent interactions—such as hydrogen bonding, π–π stacking, and hydrophobic effects—that selectively stabilize one transition state over another. Together, these strategies illustrate the conceptual breadth and growing sophistication of asymmetric C(sp3)–H functionalization. Innovations in ligand design, co-catalysis, and mechanistic understanding continue to expand the range of achievable transformations. The ability to convert simple hydrocarbons directly into complex chiral architectures underscores the growing importance of this field in modern synthesis and highlights the need for a dedicated review.
Previous surveys of transition metal-catalyzed C–H activation have largely emphasized C(sp2)–H functionalization or provided broad overviews across multiple catalytic systems. In contrast, this article focuses specifically on asymmetric C(sp3)–H functionalization mediated by CpxM(III) catalysts. The discussion is organized by transition metal (Co, Rh, Ir), with emphasis on ligand design, mechanistic paradigms, and emerging synthetic applications. By consolidating recent advances and highlighting key challenges, this review aims to capture the state of the art and provide a framework for future developments in this rapidly advancing field.
2 CpxRh(III)-catalyzed asymmetric C(sp3)–H functionalization
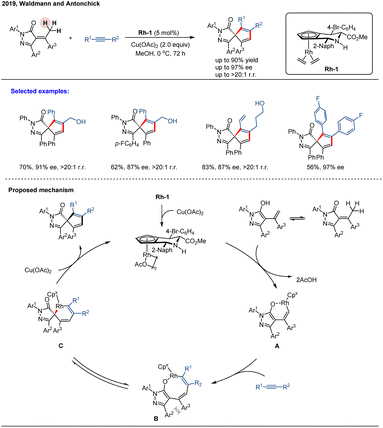
In 2019, Waldmann and co-workers reported an enantioselective annulation of α-arylidene pyrazolones with alkynes via a formal C(sp3)–H activation, catalyzed by a piperidine-fused chiral CpxRh(III) catalyst Rh-1 (Scheme 3).57 This method provided access to a diverse array of spiropyrazolones in high yields and excellent enantioselectivities (up to 97% ee). Mechanistically, tautomerization of the pyrazolone generates its enol form, which undergoes formal C(sp3)–H activation to form a six-membered rhodacycle intermediate A. The subsequent migratory insertion is regioselective for the alkyne carbon adjacent to the alkyl substituent, leading to an eight-membered rhodacycle intermediate B. Due to steric interactions between the Ar2 and Ar3 substituents, intermediate B isomerizes to intermediate C, which then undergoes reductive elimination to furnish the spiropyrazolone as a single regioisomer. The catalytic cycle is completed by reoxidation of Rh(I) to Rh(III) using Cu(OAc)2 as an oxidant. However, O2 inhibited the catalysis under otherwise identical conditions, suggesting it acts as a potent catalyst poison, likely by quenching intermediates or causing oxidative decomposition. This transformation is applicable to the late-stage diversification of chiral pharmaceuticals and natural products. The process was also demonstrated to proceed in a catalyst-directed manner with high diastereoselectivity (>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 d.r.), affording a single diastereoisomer. Beyond their synthetic utility, the spiropyrazolones obtained through this method exhibit biological activity, showing preliminary promise as a new class of Hedgehog pathway inhibitors.
5 d.r.), affording a single diastereoisomer. Beyond their synthetic utility, the spiropyrazolones obtained through this method exhibit biological activity, showing preliminary promise as a new class of Hedgehog pathway inhibitors.
 | ||
| Scheme 3 Rh(III)-catalyzed asymmetric annulation of α-arylidene pyrazolones via C(sp3)–H activation. | ||
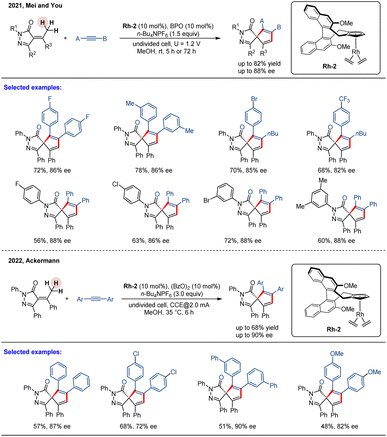
While stoichiometric oxidants remain a common necessity in catalysis, electric current offers a clean and sustainable alternative. This powerful strategy has recently been extended to metal-catalyzed enantioselective C–H functionalization, merging the principles of green chemistry with asymmetric catalysis to achieve challenging transformations under mild conditions.58 In 2021, Mei, You, and co-workers reported a highly efficient, electrochemically tuned rhodium-catalyzed system for the enantioselective formal C(sp3)–H annulation of pyrazolones with alkynes (Scheme 4).59 This operationally simple method, conducted in an undivided cell at room temperature, efficiently furnished a diverse array of spiropyrazolones in high yields (up to 82%) and with good enantiocontrol (up to 88% ee). The reaction employs a chiral BINOL-derived rhodium complex (Rh-2) as a precatalyst and benzoyl peroxide (BPO) as a key oxidative additive, with a reticulated vitreous carbon (RVC) anode and platinum cathode completing the electrochemical system (Scheme 4). In contrast to Waldmann's work, the reaction proceeds via a similar pathway; however, in this system, the CpxRh(III) catalyst is regenerated through anodic oxidation, eliminating the need for a stoichiometric chemical oxidant. Notably, this method accommodates unsymmetrical alkylarylacetylenes, which undergo smooth spiroannulation to deliver products with outstanding regiocontrol (>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 r.r.) and enantioselectivities.
5 r.r.) and enantioselectivities.
Almost simultaneously, a similar study was disclosed by Ackermann and co-workers.60 They reported an electrooxidative, enantioselective rhodium(III)-catalyzed [3 + 2] spiroannulation of pyrazolones with alkynes via C(sp3)–H activation, using a chiral BINOL-derived rhodium complex (Rh-2) as a precatalyst (Scheme 4). This protocol employs electricity as a green oxidant under mild conditions, replacing stoichiometric chemical oxidants and generating molecular hydrogen (H2) as the sole byproduct. Using a simple undivided cell, this approach provided direct access to an array of spirocyclic spiropyrazolones in moderate yield and with high enantioselectivities (up to 90% ee). Although this catalytic system exhibits good functional group tolerance with symmetrical alkynes, unsymmetrically substituted alkynes were found to be unreactive under these conditions. Note that by successfully merging electrochemistry with asymmetric catalysis, these developments provide powerful new strategies for C–H functionalization and complex molecule synthesis.
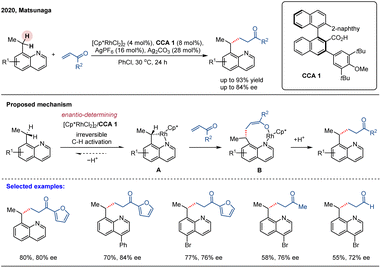
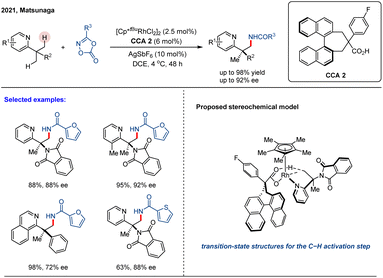
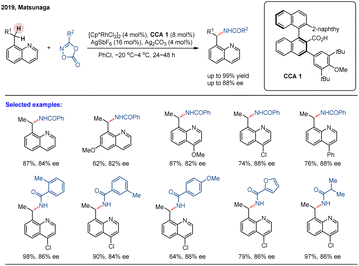
Although chiral CpxRh(III) complexes have garnered significant attention as privileged catalysts for asymmetric C–H functionalization, achiral CpxRh(III)/chiral carboxylic acid (CCA) cooperative systems have emerged as a powerful and complementary strategy.49 In 2019, Matsunaga and colleagues reported a catalytic enantioselective directed amidation of methylene C(sp3)–H bonds in 8-alkylquinolines with dioxazolones as the amidation reagent under a Cp*Rh(III)/chiral carboxylic acid hybrid catalytic system (Scheme 5).61 The quinoline moiety served as a directing group to enable chemoselective C(sp3)–H activation, while the chiral carboxylic acid ligand controlled the enantioselective cleavage of the methylene C(sp3)–H bonds, leading to the stereoselective formation of a new C–N bond at the stereogenic center. This method provided direct and efficient access to enantioenriched amide derivatives under mild reaction conditions, exhibiting both high reaction efficacy and enantioselectivities (up to 88% ee). In this study, the authors conducted a systematic evaluation of various chiral carboxylic acids (CCAs) and identified binaphthyl-derived analogues as the most effective ligand class, initially achieving an enantiomeric ratio (er) of 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32. Further structural refinement of the binaphthyl scaffold demonstrated that introducing ortho-substituents on the aryl group adjacent to the carboxylic acid significantly improved stereochemical control. Notably, employing CCA 1—featuring a 3,5-di-tert-butyl-4-methoxyphenyl substituent—under optimized conditions afforded the target γ-lactam product in 83% yield with 92
32. Further structural refinement of the binaphthyl scaffold demonstrated that introducing ortho-substituents on the aryl group adjacent to the carboxylic acid significantly improved stereochemical control. Notably, employing CCA 1—featuring a 3,5-di-tert-butyl-4-methoxyphenyl substituent—under optimized conditions afforded the target γ-lactam product in 83% yield with 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 er (Scheme 5). The practical value of this methodology was underscored by the efficient, modular synthesis of the binaphthyl-based CCAs from commercially available BINOL in five steps. This work demonstrates the synergistic combination of achiral Cp*Rh(III) and readily tunable chiral carboxylic acids as an efficient asymmetric catalytic platform for the challenging enantioselective C(sp3)–H functionalization.
8 er (Scheme 5). The practical value of this methodology was underscored by the efficient, modular synthesis of the binaphthyl-based CCAs from commercially available BINOL in five steps. This work demonstrates the synergistic combination of achiral Cp*Rh(III) and readily tunable chiral carboxylic acids as an efficient asymmetric catalytic platform for the challenging enantioselective C(sp3)–H functionalization.
 | ||
| Scheme 5 Enantioselective C(sp3)–H amidation of 8-alkylquinolines enabled by Cp*Rh(III)/CCA catalysis. | ||
As an intriguing extension of their work, Matsunaga group disclosed an asymmetric alkylation of 8-ethylquinolines with α,β-unsaturated carbonyl compounds by integrating C(sp3)–H activation with subsequent C–C bond formation (Scheme 6).62 The reaction proceeded under mild conditions with good functional group tolerance but afforded modest enantioselectivities (74–84% ee). The chiral acid scaffold is the key determinant of enantioselectivity in this system; therefore, the development of next-generation ligands constitutes the most viable path to achieving higher levels of enantiocontrol. H/D exchange experiments indicated that the C–H activation step is irreversible. Meanwhile, 18% deuterium incorporation was detected at the α-position of the carbonyl group in the product, suggesting the formation of an enolate intermediate. Overall, the mechanism studies indicate that CCA 1 mediates the enantioselective C–H activation step, thereby irreversibly generating metallacycle A in this catalytic system. Subsequent insertion of the enone and tautomerization of the resulting enol generates a nine-membered rhodacycle intermediate B. This intermediate then undergoes further protonation by the proton generated in the C–H activation step, thereby furnishing the final alkylated product and regenerating the active catalyst. Notably, substrates with 8-propyl and 8-pentyl substituents exhibited significantly lower reactivity under the standard conditions.
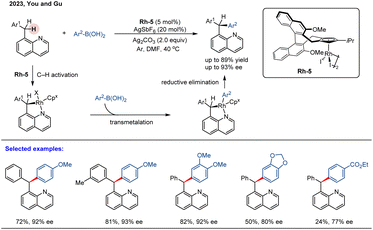
In 2023, You and co-workers reported an asymmetric benzylic C(sp3)–H arylation of 8-benzylquinolines with arylboronic acids (Scheme 7).63 Employing a BINOL-derived chiral cyclopentadienyl rhodium complex Rh-5 as the catalyst, the benzylic C(sp3)–H arylation proceeded efficiently to produce a series of enantioenriched triarylmethanes in high yields (up to 89%) with outstanding enantioselectivities (up to 93% ee). The catalytic system demonstrates broad substrate scope and excellent functional group tolerance under mild reaction conditions, providing a practical approach to valuable chiral triarylmethane scaffolds. The reaction commences with the irreversible, quinoline-directed C–H activation, forming a key rhodacyclic intermediate. This intermediate then undergoes transmetalation with the arylboronic acid, followed by reductive elimination to deliver the final product. The mechanistic study results demonstrate that the C–H activation step operates through an irreversible and enantio-determining pathway.
In 2021, Matsunaga and co-workers developed a series of pseudo-C2-symmetric chiral carboxylic acids (CCAs) based on a binaphthyl backbone, which were successfully applied in the enantioselective C(sp3)–H amidation of gem-dimethyl-substituted pyridine substrates (Scheme 8).64 The combination of sterically hindered Cp*tBuRh(III) and an optimal CCA 2 exhibits high enantiocontrol in this desymmetrization process (up to 92% ee). This transformation successfully accommodated both N-methylbenzimidazole and isoquinoline derivatives, delivering the desired products in high yields and enantioselectivities. In contrast to C1-symmetric analogues, these pseudo-C2-symmetric CCAs possess restricted conformational flexibility arising from the 2,2′-quaternary carbon bridge in their binaphthyl framework, thereby enforcing a well-defined chiral environment. Notably, a library of tunable chiral carboxylic acid ligands could be synthesized in two or three steps, which would enable rapid optimization of other valuable reactions. Mechanistic insights derived from DFT calculations and conformational analysis suggested that noncovalent interactions, such as π–π stacking or C–H–π interactions between the naphthyl moiety of CCA 2 and the pyridine moity, played a decisive role in the enantio-induction process (Scheme 8).
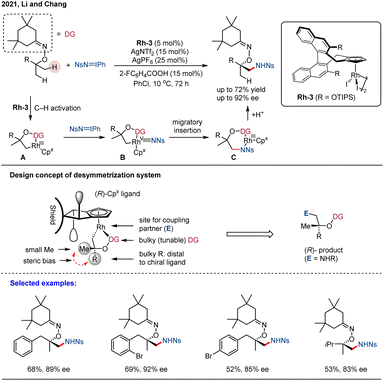
In 2021, Li and co-workers reported an enantioselective CpxRh(III)-catalyzed amination of geminal dimethyl containing compounds, affording a series of β-amino alcohol derivatives in up to 92% ee (Scheme 9).65 The enantioselective C(sp3)–H amination was accessed by a desymmetrization strategy using iminoiodinane as the N-source. Other N-sources such as TsN3 or dioxazolone were proven inactive in this transformation. While this catalytic system tolerates a diverse range of substrates, the enantioselectivity is critically modulated by the presence of an appropriate oxime directing group, a bulkier R substituent (such as tBu-, Ar-, Bn-), and a judiciously selected aminating reagent. Mechanistic analysis indicates that steric repulsion between the ligand and substrate governs both substrate orientation and transition state stabilization, leading to the high enantioselectivity. Using Rh-3 as a catalyst, a bulky directing group (oxime) was employed to assist stereodetermining C–H activation by spatially biasing one methyl group closer to the chiral pocket of the catalyst, thereby forming a rhodacycle intermediate A. Subsequently, RhV–nitrene formation (intermediate B) and migratory insertion into the nitrene generates intermediate C, which is followed by protonolysis to afford the final amination product. Studies involving H/D exchange and the kinetic isotope effect (KIE) reveal that methyl C–H activation is an irreversible and turnover-limiting step. This work highlights both the potential and the challenges of achieving high stereocontrol in simple hydrocarbon motifs.
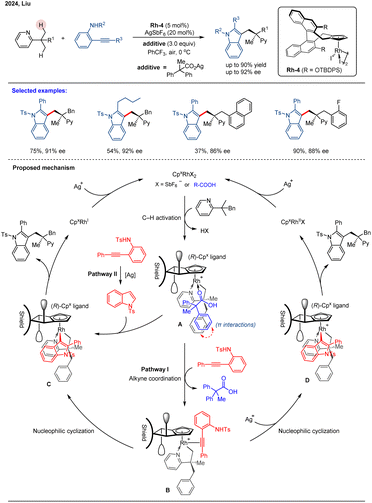
In 2024, Liu and co-workers reported a Rh(III)-catalyzed enantioselective C(sp3)–H heteroarylation of gem-dimethyl-substituted pyridine derivatives via a desymmetrization strategy.66 This reaction employs an in situ-generated arylating reagent, formed by the nucleophilic cyclization of an o-aminoaryl alkyne. Using Rh-4 as a catalyst, this catalytic system achieves outstanding enantiocontrol (up to 92% ee) in the synthesis of chiral indoles with all-carbon quaternary stereocenters (Scheme 10). The reaction was proposed to be initiated by carboxylate assisted methyl C–H activation of pyridine to afford rhodacycle intermediate A. Then, intermediate A undergoes coordination with ortho-aminoaryl alkyne (intermediate B) and in situ nucleophilic cyclization to generate Rh–aryl species D (pathway I). Then, the chiral indole product and a Rh(I) species are released by reductive elimination. Finally, the active Rh(III)-catalyst is regenerated by the oxidation of Ag(I). The in situ nucleophilic cyclization and indole formation mediated by Rh(III) species and subsequent Rh–aryl bond formation proved essential for high reaction efficiency. Alternatively, the chiral product could be afforded via pathway II, involving a NTs-indole intermediate generated from an ortho-aminoaryl alkyne through Ag-assisted nucleophilic cyclization. However, this pathway proved to be significantly less efficient. Notably, the ((2,2-diphenylpropanoyl)oxy)silver additive was crucial for achieving high stereoselectivity. Unlike previous approaches that relied on steric differentiation from bulky directing groups, stereocontrol in this system is achieved through a synergistic mechanism. Mechanistic and DFT studies reveal that the large side arm of the chiral Cpx ligand (bearing an –OTBDPS group), combined with non-covalent interactions—such as C–H⋯π and lone pair⋯π (LP⋯π) interactions between the carboxylate additive and the substrate—plays key roles in orienting the substrate within the chiral pocket of the catalyst.
The unique properties of the saturated cyclobutane ring make it a valuable structural motif in pharmaceutical chemistry. Recently, Dixon67 and co-workers reported an enantioselective amidation of cyclobutanes bearing a coordinating azine-type directing group, catalyzed by an electron-deficient CpxRh(III) complex in combination with a novel axially chiral carboxylic acid (Scheme 11).67 A broad range of substituted dioxazolones were compatible with this transformation, affording the desired products in moderate to excellent enantioselectivities (up to 98% ee) and yields (up to 94%). This method was also applied to homologous cyclohexyl and cycloheptyl substrates, providing the corresponding products in moderate yields with good enantioselectivities. In contrast, the catalytic system exhibited much diminished reactivity with cyclopentyl and cyclopropyl analogs. Furthermore, an enantioenriched bis-amidated pyridylcyclobutane was accessible following minor optimization of the reaction parameters. The proposed mechanism begins with the coordination of the pyrimidine and chiral acid co-catalyst to the Cp*Rh(III) center, followed by activation of the adjacent cyclobutyl C–H bond via an ambiphilic metal–ligand activation/concerted metalation-deprotonation (AMLA/CMD) mechanism. The kinetic isotope effect study showed that C–H bond cleavage occurs in the rate-determining step. Combining mechanistic studies with DFT calculations, the authors elucidated the origin of the enantioselectivity. Notably, the novel axially chiral carboxylic acid CCA 3, designed based on prior work by the Shi group,68 proved crucial for achieving high enantiocontrol. The fine-tuned catalytic system achieves high selectivity by stabilizing the ligand conformation through non-covalent interactions. The resulting conformation creates steric repulsion with the substrate, which induces ring strain in the transition state that leads to the minor enantiomer, thereby suppressing its formation.
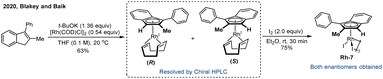
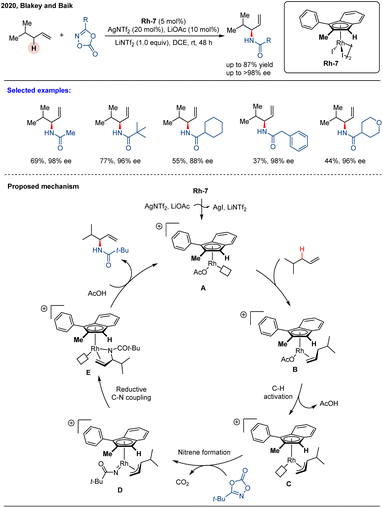
Despite the success of chiral CpxM(III) complexes in asymmetric catalysis, their enantiocontrol typically relies on steric shielding provided by judicious ligand design. In 2020, Blakey and co-workers introduced a novel class of planar chiral indenyl–rhodium(III) complexes Rh-7 that achieved enantiocontrol by exploiting electronic asymmetry rather than conventional steric bias.69 Remarkably, these chiral indenyl–Rh catalysts could be synthesized in only four steps from commercially available starting materials. Importantly, chiral HPLC purification was required to obtain the enantiopure complexes (Scheme 12). They further applied this catalytic system to the asymmetric allylic C–H amidation of unactivated terminal olefins using dioxazolones as effective amidating reagents, affording a wide array of enantioenriched allylic amides with high yields (up to 87%), excellent regioselectivity (up to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 r.r.), and outstanding enantioselectivities (up to >98% ee). The catalytic system was less reactive with internal olefin substrates, although it maintained high enantioselectivity. The proposed catalytic cycle is illustrated in Scheme 13. The reaction begins with the generation of a coordinatively unsaturated cationic indenyl–Rh complex Avia activation of the dimeric precatalyst by AgNTf2 and LiOAc. Subsequent coordination of the olefin produces intermediate B, which undergoes rate- and enantio-determining allylic C–H cleavage to form a π–allyl complex C. Coordination of the dioxazolone and loss of CO2 yields a reactive nitrene intermediate D, which undergoes regio-determining C–N bond formation to generate complex E. Finally, protodemetalation of E furnishes the desired allylic amide product and regenerates the active species A. Crystallographic analyses and computational studies revealed that both regio- and enantioselectivity are governed by a synergistic combination of electronic asymmetry and steric interactions imparted by the indenyl ligand framework.
1 r.r.), and outstanding enantioselectivities (up to >98% ee). The catalytic system was less reactive with internal olefin substrates, although it maintained high enantioselectivity. The proposed catalytic cycle is illustrated in Scheme 13. The reaction begins with the generation of a coordinatively unsaturated cationic indenyl–Rh complex Avia activation of the dimeric precatalyst by AgNTf2 and LiOAc. Subsequent coordination of the olefin produces intermediate B, which undergoes rate- and enantio-determining allylic C–H cleavage to form a π–allyl complex C. Coordination of the dioxazolone and loss of CO2 yields a reactive nitrene intermediate D, which undergoes regio-determining C–N bond formation to generate complex E. Finally, protodemetalation of E furnishes the desired allylic amide product and regenerates the active species A. Crystallographic analyses and computational studies revealed that both regio- and enantioselectivity are governed by a synergistic combination of electronic asymmetry and steric interactions imparted by the indenyl ligand framework.
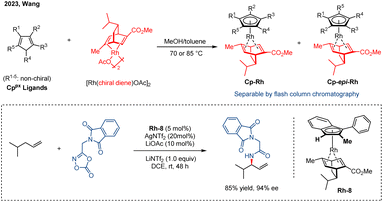
Given the persistent challenge of resolving planar chiral CpxRh(III) complexes, Wang and co-workers developed a practical and robust chiral resolution strategy.70 In this case, the chiral precursor [Rh(chiral diene)OAc]2 was synthesized in three steps from the readily available (−)-α-phellandrene. Subsequent ligand exchange with various Cpx ligands afforded a pair of air-stable CpxRh(chiral diene) diastereomers, which could be readily separated by silica gel flash chromatography (Scheme 14). This strategy enables the efficient and scalable preparation of a diverse set of tetrasubstituted planar chiral indenyl ligands without the need for extensive chiral HPLC purification or auxiliary-based resolution. The utility of these chiral indenyl–Rh(III) complexes was demonstrated across a range of asymmetric C–H functionalization reactions, notably including the allylic C–H amidation of an unactivated terminal olefin with excellent reactivity and enantioselectivity (85% yield and 94% ee).
Recently, Wang and coworkers reported a chiral CpxRh(III)-catalyzed asymmetric allylic C–H amination of unactivated terminal alkenes using sulfonamides as readily accessible nitrogen sources (Scheme 15).71 Employing CpxRh(III) catalyst Rh-6, this transformation establishes a robust platform for synthesizing chiral allylic amines in excellent yields (up to 99%) with exceptional regioselectivity (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 B/L) and high enantioselectivity (up to 96% ee). In this system, sulfonamides are converted in situ by PhI(OPiv)2 into reactive iminoiodinane species, which serve as the key nitrene precursors. The reaction scope extends to internal alkenes, as demonstrated with (E)-4-octene, which afforded two regioisomers in a 1
1 B/L) and high enantioselectivity (up to 96% ee). In this system, sulfonamides are converted in situ by PhI(OPiv)2 into reactive iminoiodinane species, which serve as the key nitrene precursors. The reaction scope extends to internal alkenes, as demonstrated with (E)-4-octene, which afforded two regioisomers in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio with moderate enantioselectivity. A proposed mechanism begins with allylic C–H activation to form a metal–allyl intermediate II. This intermediate is intercepted by the in situ-generated iminoiodinane, yielding the allyl–Rh–nitrenoid species IV. Subsequent C–N reductive coupling forms complex V, and final protodemetalation releases the chiral amide product. Mechanistic studies indicate that the allylic C–H bond cleavage is irreversible and the rate-determining step, and is also critical for enantiocontrol.
3 ratio with moderate enantioselectivity. A proposed mechanism begins with allylic C–H activation to form a metal–allyl intermediate II. This intermediate is intercepted by the in situ-generated iminoiodinane, yielding the allyl–Rh–nitrenoid species IV. Subsequent C–N reductive coupling forms complex V, and final protodemetalation releases the chiral amide product. Mechanistic studies indicate that the allylic C–H bond cleavage is irreversible and the rate-determining step, and is also critical for enantiocontrol.
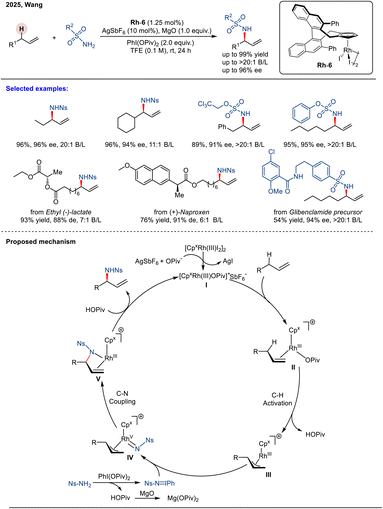 | ||
| Scheme 15 Rh(III)-catalyzed enantioselective intermolecular allylic C–H amination of unactivated alkenes. | ||
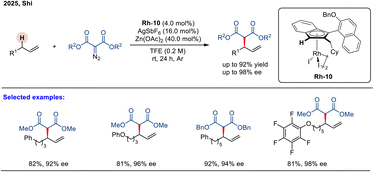
Very recently, Shi and coworkers developed a novel class of planar chiral AtroInd–rhodium(III) complexes (atropisomeric indenyl-derived rhodium(III) complexes) that enabled the asymmetric allylic C–H amination of unactivated terminal alkenes using sulfonamides as readily available nitrogen sources (Scheme 16).72 This transformation represents a significant advance in the field of allylic C–H amination. With the CpxRh(III) catalyst Rh-10, the reaction proceeds smoothly at 65 °C to furnish a wide range of enantioenriched allylic amines in excellent yields (up to 98%) and outstanding enantioselectivities (up to 99% ee).
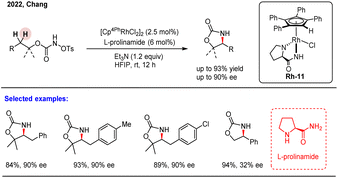
In general, CpxM(III)-catalyzed amidation reactions via the nitrene transfer pathway follow an inner-sphere or outer-sphere mechanism.34 While inner-sphere amidation forms a metalacyclic intermediate with the assistance of a directing group, the outer-sphere mechanism involves an external ligand engaged C–H insertion or stepwise hydrogen atom transfer (HAT) and C–N bond formation process, thereby providing an opportunity to induce enantioselectivity. In 2022, Chang and co-workers developed a multidimensional screening platform for the rapid in situ generation of half-sandwich metal complexes, enabling simultaneous evaluation of metal centers, CpxRh precatalysts, co-ligands, and nitrene precursors.56 This approach facilitated the efficient identification of optimal CpxRh(III)-co-ligand catalysts for both intra- and intermolecular C–H amidation reactions. Moreover, the high-throughput strategy was successfully extended to the development of a chiral catalytic system for enantioselective intramolecular amidation using N-tosyloxycarbamates as nitrene precursors. The optimal catalytic system for asymmetric intramolecular C–H amidation was identified as the in situ generated complex Rh-11, comprising an achiral Cp4Ph ligand and a chiral L-prolinamide co-ligand. This system afforded chiral 2-oxazolidinones (four examples) in good yields with high enantioselectivities (up to 90% ee, Scheme 17). Although excellent stereocontrol was achieved, the substrate scope evaluation revealed that high enantioselectivity is strongly dependent on the structure of the substituents and particularly sensitive to structural variations. This limitation highlights the need for the development of more versatile and efficient chiral rhodium catalysts for this class of asymmetric C–H amidation.
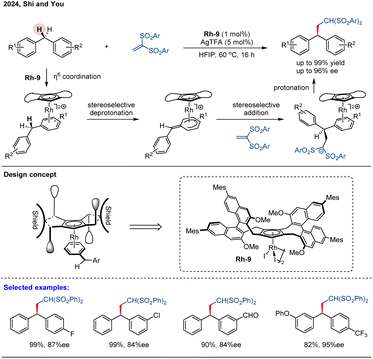
In a general asymmetric induction model, chiral Cp ligands could provide a semi-enclosed environment through the chiral backbone (back wall) and substituents on the Cp ligand (side wall). However, these chiral Cp ligands were less efficient for π-coordination-enabled benzylic C–H bond functionalization, because ligated arene and/or the C(aryl)–C(benzylic) bond can rotate, and the benzylic position is distal to these ligands. In 2024, Shi, You and co-workers developed a novel class of chiral Cp ligands featuring two identically substituted binaphthyl groups.73 By using Rh-9 as the catalyst, they achieved the asymmetric C–H bond activation of diarylmethanes bearing meta- and/or para-differentiated aryl motifs to 1,1-bis(arylsulfonyl)ethylenes in high yields (up to 99%) and excellent enantioselectivity (up to 96% ee) (Scheme 18). The authors anticipated that coordination of one arene ring of the diarylmethane to the Rh-9 center forms a rigid complex, where the sterically confined pocket constructed by the Cp ring and two nonparallel naphthyl walls restricts rotation of the bound arene ring. This spatial constraint facilitates stereoselective functionalization of the benzylic C–H bonds via a sequential arene-selective η6 coordination, stereoselective deprotonation, and stereoselective addition process, ultimately affording chiral diarylmethanes.
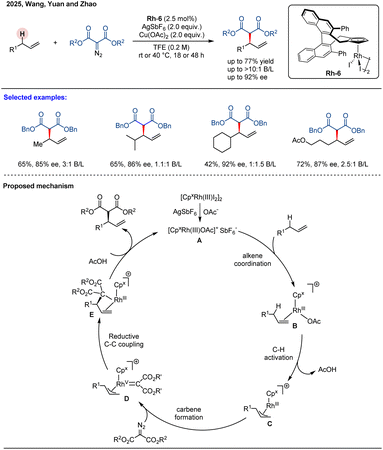
In 2025, Wang, Yuan, and co-workers developed an asymmetric allylic C–H alkylation of unactivated alkenes with α-diazocarbonyl compounds using a BINOL-derived chiral cyclopentadienyl rhodium catalyst Rh-6 (Scheme 19).74 This method provides efficient access to chiral products with good yields (up to 77%), high enantioselectivity (up to 92% ee), and regiocontrol (>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 B/L ratio). The reaction demonstrates broad substrate scope, accommodating both aliphatic and aryl-substituted terminal alkenes. Competitive and parallel KIE experiments indicate that the allylic C–H bond cleavage is the rate-determining step in the catalytic cycle. The reaction is initiated by the formation of a cationic CpxRh complex A, which is generated through activation of the dimeric precatalyst by AgSbF6 and Cu(OAc)2. Subsequently, coordination of the alkene to complex A gives intermediate B, which then undergoes a rate- and enantio-determining allylic C–H activation to afford the π–allyl Rh(III) species C. Following this, reaction with the diazo compound leads to the formation of a Rh(V)–carbene intermediate D. Next, reductive elimination from D furnishes complex E, which finally undergoes protonolysis to release the chiral product and regenerate the catalytically active Rh(III) species. Although this work constitutes an advance in asymmetric allylic C–H functionalization, the modest enantioselectivity and B/L selectivity for some substrates highlight areas for further optimization to broaden its applicability.
1 B/L ratio). The reaction demonstrates broad substrate scope, accommodating both aliphatic and aryl-substituted terminal alkenes. Competitive and parallel KIE experiments indicate that the allylic C–H bond cleavage is the rate-determining step in the catalytic cycle. The reaction is initiated by the formation of a cationic CpxRh complex A, which is generated through activation of the dimeric precatalyst by AgSbF6 and Cu(OAc)2. Subsequently, coordination of the alkene to complex A gives intermediate B, which then undergoes a rate- and enantio-determining allylic C–H activation to afford the π–allyl Rh(III) species C. Following this, reaction with the diazo compound leads to the formation of a Rh(V)–carbene intermediate D. Next, reductive elimination from D furnishes complex E, which finally undergoes protonolysis to release the chiral product and regenerate the catalytically active Rh(III) species. Although this work constitutes an advance in asymmetric allylic C–H functionalization, the modest enantioselectivity and B/L selectivity for some substrates highlight areas for further optimization to broaden its applicability.
Very recently, Shi and coworkers disclosed a related strategy for the asymmetric allylic C–H alkylation of terminal olefins, utilizing diazo compounds as convenient alkylating agents (Scheme 20).75 The deployment of a newly designed chiral AtroInd–rhodium(III) catalyst system enabled remarkable command over chemoselectivity, regioselectivity, and enantioselectivity. This catalytic system proved broadly applicable, delivering a wide scope of enantioenriched products in excellent yields (up to 92%) and with high enantiomeric excess (up to 98% ee). Mechanistically, competitive and parallel kinetic isotope effect (KIE) studies identified allylic C–H bond cleavage as the rate-determining event.
3 CpxCo(III)-catalyzed asymmetric C(sp3)–H functionalization
High-valent Cp*Co(III) complexes have garnered significant attention due to their earth abundance, low cost, and notable reactivity in C–H functionalization. In 2017, Dixon, Seayad and co-workers reported a Cp*Co(III)-catalyzed C(sp3)–H amidation of thioamides using dioxazolones as amidating agents.76 Computational studies revealed that the key cyclometalation step proceeds via an external carboxylate-assisted concerted metalation-deprotonation (CMD) mechanism, shedding light on the mechanistic features governing reactivity and selectivity in this transformation. Inspired by the seminal work of Dixon and Seayad, Matsunaga and co-workers reported an achiral CpxCo(III)/chiral carboxylic acid hybrid catalytic system for the asymmetric C(sp3)–H amidation of thioamides, employing a desymmetrization strategy.77 In this study, the authors evaluated several readily available amino acid-derived chiral carboxylic acids (CCAs) as ligands. The tert-leucine-derived CCA 4 was identified as optimal. Furthermore, modification of the cobalt catalyst revealed that the more sterically hindered Cp*tBuCo(III) complex provided the highest enantioselectivity, albeit with a slight reduction in reactivity. A series of chiral β-amino thiocarbonyl building blocks containing quaternary stereocenters were successfully synthesized, achieving moderate to good enantioselectivities of up to 88% ee (Scheme 21). Mechanistically, the active species CpxCo(O2CR)+ may form between CpxCo(III) and the chiral acid co-catalyst CCA 4, possibly aided by the basic substrates. Subsequent binding of the thioamide substrate generates intermediate B, which undergoes cyclometallation via a carboxylate-assisted concerted metalation-deprotonation (CMD) process to yield the cobaltacycle intermediate D. This C–H activation step was shown to be both irreversible and the enantioselectivity-determining step. Coordination of the dioxazolone followed by migratory insertion of the nitrenoid moiety furnishes intermediate F. Finally, protonolysis of the cobalt–carbon bond in F releases the chiral product and regenerates the active catalytic species, thereby completing the catalytic cycle.In 2019, Matsunaga group developed new planar chiral ferrocene-based carboxylic acids as chiral co-ligands for this CpxCo(III)-catalyzed enantioselective C–H amidation of thioamides (Scheme 22).78 These chiral 2-aryl ferrocene carboxylic acids (CCAs) were synthesized via a highly efficient diastereoselective ortho-lithiation/Suzuki–Miyaura cross-coupling sequence, enabling a modular and versatile ligand scaffold. Systematic screening identified the sterically encumbered ligand CCA 5 as optimal, although the system provided only moderate enantioselectivity (up to 74% ee) despite high reactivity. The authors proposed a stereochemical model wherein enantioinduction arises from minimizing steric repulsion between the substrate and the chiral ligand environment.
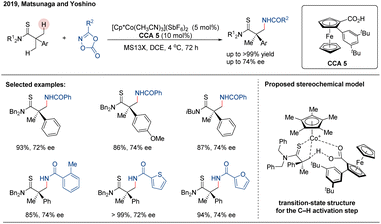 | ||
| Scheme 22 Co(III)-ferrocene carboxylic acid catalyzed enantioselective C(sp3)–H amidation of α-aryl thioamides. | ||
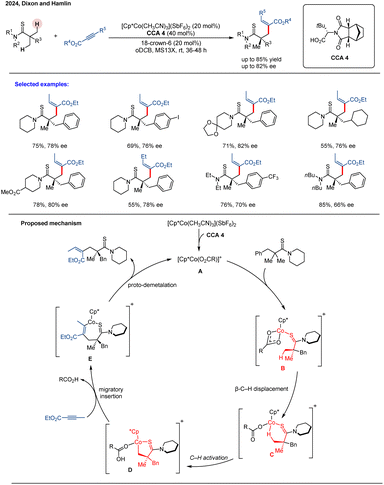
In 2024, Dixon and co-workers developed a Cp*Co(III)-catalyzed asymmetric C(sp3)–H alkenylation of thioamides using but-2-ynoate esters as coupling partners.79 The reaction utilized an achiral Cp*Co complex with a chiral carboxylic acid as the catalyst. Screening of various amino acid-derived CCAs identified tert-leucine-derived CCA 3 as optimal, providing the highest enantioselectivity. While the direct reaction gave moderate enantiomeric excess (up to 82% ee), recrystallization afforded enantiopure products (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
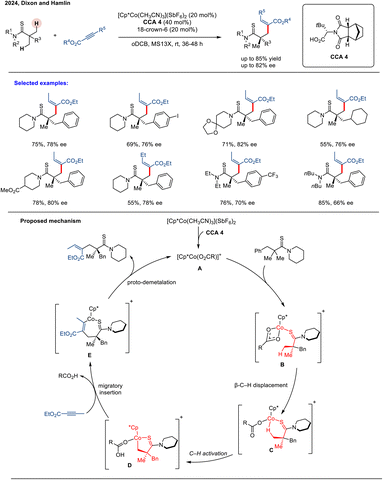
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). These enantioenriched compounds were further diversified into various valuable, complex scaffolds, demonstrating the method's synthetic utility. Complementary computational studies revealed that steric repulsions govern both enantioselectivity in the C–H activation step and regioselectivity during migratory insertion (Scheme 23). The catalytic cycle begins with thioamide coordination to form complex B. C–H activation then proceeds via a κ2-to-κ1 carboxylate displacement by the substrate's β-hydrogen (C), following a CMD mechanism to generate cobaltacycle D. A steric clash between the chiral co-catalyst's tBu group and the substrate's benzyl group plays a critical role in establishing stereocontrol in this step. Subsequent alkyne migratory insertion affords intermediate E, and protonolysis releases the product while regenerating the active Cp*Co(O2CR)+ catalyst. Despite these advances, the requirement for a high catalyst loading (20 mol%) could hinder practical scalability. This limitation highlights the necessity for developing more active and robust chiral CpxCo(III) catalytic systems capable of operating at lower loadings while providing enhanced asymmetric induction.
1 er). These enantioenriched compounds were further diversified into various valuable, complex scaffolds, demonstrating the method's synthetic utility. Complementary computational studies revealed that steric repulsions govern both enantioselectivity in the C–H activation step and regioselectivity during migratory insertion (Scheme 23). The catalytic cycle begins with thioamide coordination to form complex B. C–H activation then proceeds via a κ2-to-κ1 carboxylate displacement by the substrate's β-hydrogen (C), following a CMD mechanism to generate cobaltacycle D. A steric clash between the chiral co-catalyst's tBu group and the substrate's benzyl group plays a critical role in establishing stereocontrol in this step. Subsequent alkyne migratory insertion affords intermediate E, and protonolysis releases the product while regenerating the active Cp*Co(O2CR)+ catalyst. Despite these advances, the requirement for a high catalyst loading (20 mol%) could hinder practical scalability. This limitation highlights the necessity for developing more active and robust chiral CpxCo(III) catalytic systems capable of operating at lower loadings while providing enhanced asymmetric induction.
4 CpxIr(III)-catalyzed asymmetric C(sp3)–H functionalization
Cyclometallated Cp*Ir(III) complexes are well-established for promoting C–H activation via inner-sphere mechanisms; however, enantioselective C(sp3)–H functionalization using such pathways remains challenging. In a 2018 breakthrough, Chang and co-workers demonstrated that Cp*Ir(III) complexes, when leveraged with electron-donating bidentate ligands, could achieve intramolecular C(sp3)–H amidation through an outer-sphere mechanism, enabling the highly selective synthesis of γ-lactams from 1,4,2-dioxazol-5-ones.80 Building on this, they subsequently developed a Cp*Ir(III)-catalyzed asymmetric intramolecular C(sp3)–H amidation for synthesizing chiral γ-lactams—privileged scaffolds prevalent in natural products and bioactive compounds (Scheme 24).54 Through systematic evaluation of N,O- and N,N′-bidentate chiral ligands, an N,N′-donor ligand (Ir-1) was identified as optimal. The reaction exhibited a broad substrate scope, efficiently converting readily accessible dioxazolones into γ-lactams in high yield with excellent enantioselectivity. It proved compatible with diverse secondary C–H bonds—including benzylic, unactivated aliphatic, propargylic, and allylic sites—delivering products with high regio- and stereocontrol. Mechanistic studies, supported by DFT calculations, indicated that hydrogen-bonding interactions between the substrate and chiral catalyst are pivotal in the enantio-determining step. Notably, the methodology was successfully applied to the desymmetrization of meso-substrates, enabling the simultaneous installation of two contiguous stereocenters in a single transformation. This work underscores the potential of outer-sphere Ir(III) catalysis for asymmetric C(sp3)–H functionalization and opens up new avenues for the precise construction of complex chiral architectures.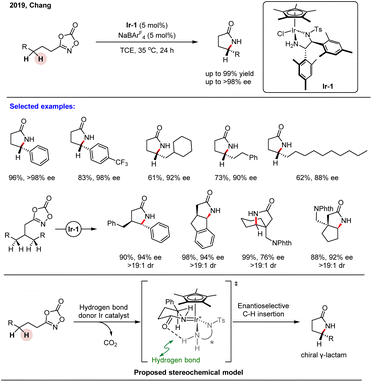 | ||
| Scheme 24 Piano-stool type Ir(III)-catalyzed asymmetric intramolecular C(sp3)–H amidation of dioxazolone. | ||
In the same year, Chen, He, Chang, and co-workers reported a highly enantioselective intramolecular C(sp3)–H amidation catalyzed by a newly designed, α-amino-acid-based ligand in combination with Cp*Ir(III).55 Through systematic ligand exploration, they identified the pre-formed, diarylated complex Ir-2—derived from an aminoquinoline (AQ)-appended chiral amide—as the optimal catalyst, which provided both high reactivity and excellent enantioselectivity (Scheme 25). This system efficiently converted dioxazolones into optically enriched γ-lactams, demonstrating broad functional group tolerance across diverse secondary C–H bonds (including benzylic, unactivated aliphatic, propargylic, and allylic sites) and proving effective in desymmetrization reactions. Mechanistic studies revealed that the Cp*, AQ, and phthalimide (Phth) groups in Ir-2 collectively generate an enzyme-like hydrophobic pocket around the iridium center. This distinctive architecture promotes efficient substrate binding and conversion, even in polar or aqueous media. Notably, enantiocontrol is achieved via a network of noncovalent interactions—primarily π–π stacking and hydrogen bonding—rather than through traditional steric repulsion. This work establishes a distinct biomimetic paradigm for enantioinduction and expands the toolbox for designing sophisticated chiral transition metal catalysts.
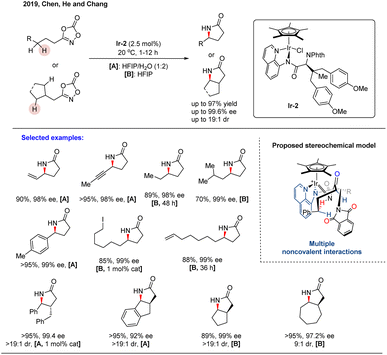 | ||
| Scheme 25 Piano-stool type Ir(III)-catalyzed enantioselective intramolecular C(sp3)–H amidation of dioxazolone. | ||
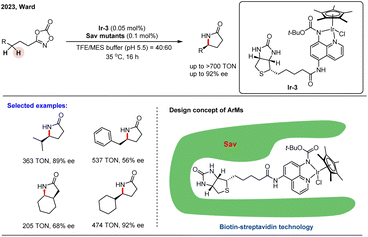
Artificial metalloenzymes (ArMs) represent a powerful class of hybrid catalysts that combine synthetic metal centers with protein scaffolds.81–83 This strategy merges the versatile reactivity of organometallic catalysis with the exquisite selectivity and evolvability of biological systems. To unlock their full potential, a synergistic approach is essential, involving precise chemical modification of the metal cofactor alongside protein engineering of the host to control catalytic activity and stereoselectivity.
A significant advance in this field was reported by Ward and co-workers, who constructed an artificial metalloenzyme for the enantioselective amidation of unactivated C(sp3)–H bonds using biotin–streptavidin (Sav) technology.84 They incorporated a series of biotinylated Cp*Ir(III) cofactors into the Sav scaffold to generate chiral catalytic ArMs. These assemblies promoted intramolecular C–H amidation to form γ-lactams with high regioselectivity and moderate enantioselectivity. The cofactor [Cp*Ir(Boc-AQ-biot)Cl] (Ir-3) showed the best initial performance, affording the product with 47 turnovers (TON) and 30% ee. To improve stereocontrol, the authors employed iterative saturation mutagenesis to evolve the protein's secondary coordination sphere. This engineering strategy yielded a significantly improved variant capable of achieving up to 308 TON and 86% ee. Subsequent substrate scope evaluation demonstrated that the optimized ArM could reach enantiomeric excesses of up to 92%, highlighting its potential as a useful platform for asymmetric C–H functionalization (Scheme 26).
5 The challenge of asymmetric C(sp3)–H functionalization with CpxM(III) (M = Co, Rh, Ir) catalysts
Although asymmetric C(sp3)–H functionalization catalyzed by CpxM(III) complexes (M = Co, Rh, Ir) has emerged as a transformative strategy in modern organic synthesis, several critical challenges continue to impede its broader application. Chief among these is the fundamental inertness of C(sp3)–H bonds, which exhibit low acidity, lack polarity, and often reside in sterically congested environments. These intrinsic properties demand the development of highly efficient, site-selective, and stereochemically defined catalytic systems capable of overcoming formidable kinetic and thermodynamic barriers.Attaining high levels of enantioselectivity remains particularly demanding. It necessitates precise control over the chiral environment at the metal center, which must be delicately tuned through rational ligand design and chiral catalyst development. The development of chiral Cpx ligands that are both broadly applicable and capable of differentiating between similar C–H bonds in complex settings is still in its infancy. The reported chiral catalysts have primarily considered different types of chiral backbones to introduce a suitable chiral environment and achieve a high level of asymmetric control. However, the chiral environment directly on the metal center—closest to the reactive site—has received less attention as a tool for tuning catalytic activity and asymmetric induction. This underexplored area presents a significant opportunity for more diverse catalyst design. In addition, the reported synthesis of chiral Cpx ligands and their corresponding metal complexes often involves multiple steps. This significantly increases their cost, hinders large-scale preparation, and limits their widespread application. Furthermore, the catalytic potential and intrinsic reactivity differences among Rh-, Ir-, and Co-based Cpx M(III) systems have not yet been systematically explored or directly compared, which presents a significant opportunity for further development. Beyond the commonly studied M(III) oxidation state, catalysts based on alternative metal oxidation states (like the M(I) oxidation state) also remain largely unexplored. These systems may offer distinct reactivity patterns and unique mechanistic pathways for asymmetric C(sp3)–H functionalization. Consequently, the development of more streamlined, modular, and scalable synthetic routes to chiral catalysts with diverse electronic and steric properties remains a critical and highly desirable objective in the field.
The substrate scope for asymmetric CpxM(III)-catalyzed C(sp3)–H functionalization is often limited by sensitivity to polar or coordinating functional groups. Furthermore, the regio- and enantioselective activation of challenging unactivated C(sp3)–H bonds (e.g., in alkanes) remains a major unsolved challenge. Furthermore, despite substantial methodological progress, the application of this strategy to the synthesis of complex natural products and pharmaceuticals remains underdeveloped. Most studies rely on simplified model substrates, while examples of late-stage functionalization of architecturally complex bioactive molecules are rare. Continued efforts in this direction will be important for fully realizing the synthetic potential of this strategy in medicinal chemistry and natural product synthesis. Overcoming these hurdles would greatly enhance the method's synthetic power.
Key mechanistic aspects—including the nature of metal–carbon bond formation, the role of concerted-metalation-deprotonation (CMD) versus outer-sphere pathways, and the origin of stereoselectivity—require further elucidation to guide rational catalyst design and enable novel, challenging transformations. In particular, a systematic, predictive understanding of how the steric and electronic parameters of CpxM(III) complexes influence catalytic performance is lacking. Dedicated studies aimed at establishing structure–activity relationships through integrated experimental and computational (DFT) approaches are needed to unlock the potential of this catalytic system in asymmetric C(sp3)–H functionalization.
Addressing these challenges will require a synergistic integration of ligand innovation, mechanistic insight, and synthetic methodology development. Emerging tools such as high-throughput experimentation, in situ spectroscopic techniques, and computational modelling hold promise for accelerating catalyst discovery and reaction optimization. Expanding the scope of compatible substrates, enhancing catalyst robustness, and simplifying operational protocols are essential next steps in establishing asymmetric C(sp3)–H functionalization as a general and practical platform for stereoselective synthesis.
6 Summary and outlook
Enantioselective C(sp3)–H functionalization mediated by group 9 CpxM(III) complexes (M = Co, Rh, Ir) has emerged as a transformative paradigm for the direct catalytic assembly of stereogenic centers. This review comprehensively examines strategic advances in catalyst design—encompassing tailored chiral cyclopentadienyl (Cpx) ligands, cooperative chiral carboxylic acid (CCA) auxiliaries, and modular bidentate coordination complexes—that have enabled remarkable regio- and enantiocontrol in the functionalization of challenging sp3-hybridized C–H bonds.While substantial progress has been made, the field continues to face fundamental challenges, including limited reaction types, narrow substrate scope, and modest catalyst efficiency. Overcoming these limitations will require deepened mechanistic insight and innovative ligand architectures, particularly for earth-abundant cobalt catalysts. The broad synthetic utility of these methods—spanning complex molecule synthesis, pharmaceutical development, and materials science—underscores their potential to redefine retrosynthetic planning.
CpxM(III)-catalyzed asymmetric C(sp3)–H functionalization is a rapidly advancing frontier in organic synthesis. This review critically analyses the field's current achievements and identifies persistent challenges to map a path toward more practical, efficient, and sustainable stereoselective strategies. Ultimately, we aim to provide not only a comprehensive overview but also to inspire further exploration, accelerating the development of scalable methods for the synthesis of complex chiral molecules.
Author contributions
Shu-Bin Mou: writing-original draft, methodology. Mu-Peng Luo: writing-review & editing, Feifei Fang: writing-review & editing. Shi Cao: writing-review & editing, supervision. Dong Wu: review, supervision, project administration. Shou-Guo Wang: supervision, project administration, funding acquisition.Conflicts of interest
The authors declare no conflict of interest.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
This work was supported by Guangdong Basic and Applied Basic Research Foundation (2024A1515011368 and 2024QN11C213); the Scientific Foundation for Youth Scholars of Shenzhen University (868-000001033009); and the National Natural Science Foundation of China (22333006). S.-G. is indebted to Shenzhen University for providing a start-up grant.References
- A. García-Viada, J. C. Carretero, J. Adrio and N. Rodríguez, Chem. Soc. Rev., 2025, 54, 4353–4390 Search PubMed.
- J. Grover, A. T. Sebastian, S. Maiti, A. C. Bissember and D. Maiti, Chem. Soc. Rev., 2025, 54, 2006–2053 Search PubMed.
- J. F. Hartwig, J. Am. Chem. Soc., 2016, 138, 2–24 CrossRef CAS PubMed.
- I. F. Yu, J. W. Wilson and J. F. Hartwig, Chem. Rev., 2023, 123, 11619–11663 Search PubMed.
- B. Liu, A. M. Romine, C. Z. Rubel, K. M. Engle and B. F. Shi, Chem. Rev., 2021, 121, 14957–15074 Search PubMed.
- S. K. Sinha, S. Guin, S. Maiti, J. P. Biswas, S. Porey and D. Maiti, Chem. Rev., 2022, 122, 5682–5841 Search PubMed.
- O. Baudoin, Chem. Soc. Rev., 2011, 40, 4902–4911 Search PubMed.
- R. Bisht, C. Haldar, M. M. M. Hassan, M. E. Hoque, J. Chaturvedi and B. Chattopadhyay, Chem. Soc. Rev., 2022, 51, 5042–5100 Search PubMed.
- L. Ping, D. S. Chung, J. Bouffard and S.-G. Lee, Chem. Soc. Rev., 2017, 46, 4299–4328 Search PubMed.
- H. Huang, X. Ji, W. Wu and H. Jiang, Chem. Soc. Rev., 2015, 44, 1155–1171 Search PubMed.
- J. A. Labinger and J. E. Bercaw, Nature, 2002, 417, 507–514 Search PubMed.
- J. H. Docherty, T. M. Lister, G. McArthur, M. T. Findlay, P. Domingo-Legarda, J. Kenyon, S. Choudhary and I. Larrosa, Chem. Rev., 2023, 123, 7692–7760 CrossRef CAS PubMed.
- B. M. Trost, Science, 1991, 254, 1471–1477 CrossRef CAS PubMed.
- C. G. Newton, S. G. Wang, C. C. Oliveira and N. Cramer, Chem. Rev., 2017, 117, 8908–8976 CrossRef CAS PubMed.
- R. Giri, B. F. Shi, K. M. Engle, N. Maugel and J. Q. Yu, Chem. Soc. Rev., 2009, 38, 3242–3272 RSC.
- H.-H. Li, X. Chen and S. Kramer, Chem. Sci., 2023, 14, 13278–13289 RSC.
- X. Yu, Z.-Z. Zhang, J.-L. Niu and B. F. Shi, Org. Chem. Front., 2022, 9, 1458–1484 Search PubMed.
- Q. Zhang, L.-S. Wu and B.-F. Shi, Chem, 2022, 8, 384–413 CAS.
- T. G. Saint-Denis, R.-Y. Zhu, G. Chen, Q.-F. Wu and J.-Q. Yu, Science, 2018, 359, eaao4798 CrossRef PubMed.
- J. Pedroni and N. Cramer, Chem. Commun., 2015, 51, 17647–17657 RSC.
- J. He, M. Wasa, K. S. L. Chan, Q. Shao and J. Q. Yu, Chem. Rev., 2017, 117, 8754–8786 CrossRef CAS PubMed.
- P. S. Wang and L. Z. Gong, Acc. Chem. Res., 2020, 53, 2841–2854 CrossRef CAS PubMed.
- K. Yang, M. Song, H. Liu and H. Ge, Chem. Sci., 2020, 11, 12616–12632 RSC.
- C.-X. Liu, S.-Y. Yin, F. Zhao, H. Yang, Z. Feng, Q. Gu and S.-L. You, Chem. Rev., 2023, 123, 10079–10134 Search PubMed.
- B. Ye and N. Cramer, Acc. Chem. Res., 2015, 48, 1308–1318 Search PubMed.
- G. Song, F. Wang and X. Li, Chem. Soc. Rev., 2012, 41, 3651–3678 RSC.
- Y. Zhang, J.-J. Zhang, L. Lou, L. Lin, N. Cramer, S.-G. Wang and Z. Chen, Chem. Soc. Rev., 2024, 53, 3457–3484 RSC.
- K. Yamakawa and T. Nishimura, Chem. Commun., 2025, 61, 13094–13108 RSC.
- R. Kaur and N. Jain, Chem. Asian J., 2022, 17, e202200944 CrossRef CAS PubMed.
- T. Nishimura, Chem. Rec., 2021, 21, 3532–3545 CrossRef CAS PubMed.
- Ł. Woźniak, J.-F. Tan, Q.-H. Nguyen, A. M. Vigné, V. Smal, Y.-X. Cao and N. Cramer, Chem. Rev., 2020, 120, 10516–10543 CrossRef PubMed.
- B. Garai, A. Das, D. V. Kumar and B. Sundararaju, Chem. Commun., 2024, 60, 3354–3369 RSC.
- Q. J. Yao and B. F. Shi, Acc. Chem. Res., 2025, 58, 9719–9990 Search PubMed.
- J. Lee and S. Chang, Synlett, 2023, 34, 1356–1366 CrossRef CAS.
- Y. Zheng, C. Zheng, Q. Gu and S. L. You, Chem Catal., 2022, 2, 2965–2985 CAS.
- P. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz and L. Ackermann, Chem. Rev., 2019, 119, 2192–2452 CrossRef CAS PubMed.
- J. Mo, A. M. Messinis, J. Li, S. Warratz and L. Ackermann, Acc. Chem. Res., 2024, 57, 10–22 CrossRef CAS PubMed.
- Ł. Woźniak and N. Cramer, Trends Chem., 2019, 1, 471–484 CrossRef.
- J. Loup, U. Dhawa, F. Pesciaioli, J. Wencel-Delord and L. Ackermann, Angew. Chem., Int. Ed., 2019, 58, 12803–12818 Search PubMed.
- X.-J. Si, T.-C. Wang, T.-P. Loh and M.-Z. Lu, Chem. Sci., 2025, 16, 5836–5848 RSC.
- Y. He, Z. Huang, K. Wu, J. Ma, Y.-G. Zhou and Z. Yu, Chem. Soc. Rev., 2022, 51, 2759–2852 Search PubMed.
- T. K. Achar, S. Maiti, S. Jana and D. Maiti, ACS Catal., 2020, 10, 13748–13793 CrossRef CAS.
- T. K. Hyster, L. Knörr, T. R. Ward and T. Rovis, Science, 2012, 338, 500–503 CrossRef CAS PubMed.
- B. Ye and N. Cramer, Science, 2012, 338, 504–506 Search PubMed.
- C. Davies, S. Shaaban and H. Waldmann, Trends Chem., 2022, 4, 318–330 CrossRef CAS.
- J. Mas-Roselló, A. G. Herraiz, B. Audic, A. Laverny and N. Cramer, Angew. Chem., Int. Ed., 2021, 60, 13198–13224 Search PubMed.
- S. Shaaban, C. Davies and H. Waldmann, Eur. J. Org Chem., 2020, 6512–6524 Search PubMed.
- T. Yoshino, S. Satake and S. Matsunaga, Chem.–Eur. J., 2020, 26, 7346–7357 CrossRef CAS PubMed.
- T. Yoshino and S. Matsunaga, ACS Catal., 2021, 11, 6455–6466 Search PubMed.
- C. Pan, S. Y. Yin, Q. Gu and S. L. You, Org. Biomol. Chem., 2021, 19, 7264–7275 Search PubMed.
- P.-F. Qian, J.-Y. Li, Y.-B. Zhou, T. Zhou and B.-F. Shi, SynOpen, 2023, 07, 466–485 CrossRef CAS.
- S. K. Sinha, P. Ghosh, S. Jain, S. Maiti, S. A. Al-Thabati, A. A. Alshehri, M. Mokhtar and D. Maiti, Chem. Soc. Rev., 2023, 52, 7461–7503 RSC.
- C. G. Newton, D. Kossler and N. Cramer, J. Am. Chem. Soc., 2016, 138, 3935–3941 CrossRef CAS PubMed.
- Y. Park and S. Chang, Nat. Catal., 2019, 2, 219–227 CrossRef CAS.
- H. Wang, Y. Park, Z. Bai, S. Chang, G. He and G. Chen, J. Am. Chem. Soc., 2019, 141, 7194–7201 CrossRef CAS PubMed.
- J. Jeong, H. Jung, D. Kim and S. Chang, ACS Catal., 2022, 12, 8127–8138 CrossRef CAS.
- H. Li, R. Gontla, J. Flegel, C. Merten, S. Ziegler, A. P. Antonchick and H. Waldmann, Angew. Chem., Int. Ed., 2019, 58, 307–311 CrossRef CAS PubMed.
- C. Ma, P. Fang and T.-S. Mei, ACS Catal., 2018, 8, 7179–7189 CrossRef CAS.
- Y.-Q. Huang, Z.-J. Wu, L. Zhu, Q. Gu, X. Lu, S.-L. You and T.-S. Mei, CCS Chem., 2022, 4, 3181–3189 CrossRef CAS.
- W. Wei, A. Scheremetjew and L. Ackermann, Chem. Sci., 2022, 13, 2783–2788 RSC.
- S. Fukagawa, M. Kojima, T. Yoshino and S. Matsunaga, Angew. Chem., Int. Ed., 2019, 58, 18154–18158 CrossRef CAS PubMed.
- L.-T. Huang, S. Fukagawa, M. Kojima, T. Yoshino and S. Matsunaga, Org. Lett., 2020, 22, 8256–8260 CrossRef CAS PubMed.
- Z.-Y. Zhang, B.-B. Gou, Q. Wang, Q. Gu and S.-L. You, Adv. Synth. Catal., 2024, 366, 774–779 CrossRef CAS.
- Y. Kato, L. Lin, M. Kojima, T. Yoshino and S. Matsunaga, ACS Catal., 2021, 11, 4271–4277 Search PubMed.
- B. Liu, P. Xie, J. Zhao, J. Wang, M. Wang, Y. Jiang, J. Chang and X. Li, Angew. Chem., Int. Ed., 2021, 60, 8396–8400 Search PubMed.
- Y. Shi, Y. Qiao, P. Xie, M. Tian, X. Li, J. Chang and B. Liu, Chin. Chem. Lett., 2024, 35, 109544 CrossRef CAS.
- X. Xu, H. Shi, D.-H. Tan, P. Biallas, A. J. M. Farley, C. Genicot, K. Yamazaki and D. J. Dixon, Chem Catal., 2025, 5, 101413 Search PubMed.
- T. Zhou, P.-F. Qian, J.-Y. Li, Y. B. Zhou, H.-C. Li, H.-Y. Chen and B.-F. Shi, J. Am. Chem. Soc., 2021, 143, 6810–6816 Search PubMed.
- C. M. B. Farr, A. M. Kazerouni, B. Park, C. D. Poff, J. Won, K. R. Sharp, M.-H. Baik and S. B. Blakey, J. Am. Chem. Soc., 2020, 142, 13996–14004 Search PubMed.
- C. Zhang, J. Jiang, X. Huang and J. Wang, ACS Catal., 2023, 13, 10468–10473 CrossRef CAS.
- Y.-Y. Liu, S. Cao, M.-P. Luo and S.-G. Wang, CCS Chem., 2025 DOI:10.31635/ccschem.025.202506179.
- Y. Zheng, J. Zhang, J. Bai, M. Wang and Z. Shi, J. Am. Chem. Soc., 2025, 147, 40833–40841 Search PubMed.
- W.-Q. Wu, P.-P. Xie, L.-Y. Wang, B.-B. Gou, Y. Lin, L.-W. Hu, C. Zheng, S.-L. You and H. Shi, J. Am. Chem. Soc., 2024, 146, 26630–26638 Search PubMed.
- Q. Zhou, B. Yang, G. Hao, M. Luo, S. Cao, B. Zhao, H. Yuan and S.-G. Wang, Chin. J. Org. Chem., 2025, 45, 2109–2120 CrossRef CAS.
- Y. Zheng, F. Xiang, J. Zhang, X. Yin, M. Wang and Z. Shi, Angew. Chem., Int. Ed., 2025, e202519953 Search PubMed.
- P. W. Tan, A. M. Mak, M. B. Sullivan, D. J. Dixon and J. Seayad, Angew. Chem., Int. Ed., 2017, 56, 16550–16554 Search PubMed.
- S. Fukagawa, Y. Kato, R. Tanaka, M. Kojima, T. Yoshino and S. Matsunaga, Angew. Chem., Int. Ed., 2019, 58, 1153–1157 Search PubMed.
- D. Sekine, K. Ikeda, S. Fukagawa, M. Kojima, T. Yoshino and S. Matsunaga, Organometallics, 2019, 38, 3921–3926 Search PubMed.
- L. Staronova, K. Yamazaki, X. Xu, H. Shi, F. M. Bickelhaupt, T. A. Hamlin and D. J. Dixon, Angew. Chem., Int. Ed., 2024, 63, e202316021 CrossRef CAS PubMed.
- S. Y. Hong, Y. Park, Y. Hwang, Y. B. Kim, M. H. Baik and S. Chang, Science, 2018, 359, 1016–1021 Search PubMed.
- F. Schwizer, Y. Okamoto, T. Heinisch, Y. Gu, M. M. Pellizzoni, V. Lebrun, R. Reuter, V. Köhler, J. C. Lewis and T. R. Ward, Chem. Rev., 2018, 118, 142–231 CrossRef CAS PubMed.
- H. J. Davis and T. R. Ward, ACS Cent. Sci., 2019, 5, 1120–1136 Search PubMed.
- A. D. Liang, J. Serrano-Plana, R. L. Peterson and T. R. Ward, Acc. Chem. Res., 2019, 52, 585–595 CrossRef CAS PubMed.
- K. Yu, Z. Zou, N. V. Igareta, R. Tachibana, J. Bechter, V. Köhler, D. Chen and T. R. Ward, J. Am. Chem. Soc., 2023, 145, 16621–16629 Search PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2026 |